Characterization of phosphoglycerate kinase-1 expression of stromal cells derived from tumor microenvironment in prostate cancer progression
- PMID: 20068185
- PMCID: PMC3086494
- DOI: 10.1158/0008-5472.CAN-09-2863
Characterization of phosphoglycerate kinase-1 expression of stromal cells derived from tumor microenvironment in prostate cancer progression
Retraction in
-
Retraction: Characterization of Phosphoglycerate Kinase-1 Expression of Stromal Cells Derived from Tumor Microenvironment in Prostate Cancer Progression.Cancer Res. 2022 Sep 16;82(18):3405. doi: 10.1158/0008-5472.CAN-22-2398. Cancer Res. 2022. PMID: 36111404 Free PMC article. No abstract available.
Abstract
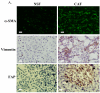
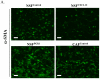
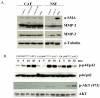
Tumor and stromal interactions in the tumor microenvironment are critical for oncogenesis and cancer progression. Our understanding of the molecular events by which reactive stromal fibroblasts-myofibroblast or cancer-associated fibroblasts (CAF)-affect the growth and invasion of prostate cancer remains unclear. Laser capture microdissection and cDNA microarray analysis of CAFs in prostate tumors revealed strong upregulation of phosphoglycerate kinase-1 (PGK1), an ATP-generating glycolytic enzyme that forms part of the glycolytic pathway and is directly involved in CXCL12-CXCR4 signaling. Normal fibroblasts overexpressing PGK1 resembled myofibroblasts in their expression of smooth muscle alpha-actin, vimentin, and high levels of CXCL12. These cells also displayed a higher proliferative index and the capability to contribute to prostate tumor cell invasion in vitro, possibly through expression of MMP-2 and MMP-3 and activation of the AKT and ERK pathways. Coimplantation of PGK1-overexpressing fibroblasts with prostate tumor cells promoted tumor cell growth in vivo. Collectively, these observations suggest that PGK1 helps support the interactions between cancer and its microenvironment.
Figures













References
-
- Concato J, Jain D, Uchio E, et al. Molecular markers and death from prostate cancer. Ann Intern Med. 2009;150:595–603. - PubMed
-
- Hida K, Hida Y, Amin DN, et al. Tumor-associated endothelial cells with cytogenetic abnormalities. Cancer Res. 2004;64:8249–55. - PubMed
-
- Micke P, Ostman A. Exploring the tumour environment: cancer-associated fibroblasts as targets in cancer therapy. Expert Opin Ther Targets. 2005;9:1217–33. - PubMed
-
- Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

