Bortezomib induces apoptosis in primitive chronic myeloid leukemia cells including LTC-IC and NOD/SCID repopulating cells
- PMID: 20068223
- PMCID: PMC2844011
- DOI: 10.1182/blood-2008-06-164582
Bortezomib induces apoptosis in primitive chronic myeloid leukemia cells including LTC-IC and NOD/SCID repopulating cells
Abstract
Chronic myeloid leukemia (CML) is treated effectively with tyrosine kinase inhibitors (TKIs); however, 2 key problems remain-the insensitivity of CML stem and progenitor cells to TKIs and the emergence of TKI-resistant BCR-ABL mutations. BCR-ABL activity is associated with increased proteasome activity and proteasome inhibitors (PIs) are cytotoxic against CML cell lines. We demonstrate that bortezomib is antiproliferative and induces apoptosis in chronic phase (CP) CD34+ CML cells at clinically achievable concentrations. We also show that bortezomib targets primitive CML cells, with effects on CD34+38(-), long-term culture-initiating (LTC-IC) and nonobese diabetic/severe combined immunodeficient (NOD/SCID) repopulating cells. Bortezomib is not selective for CML cells and induces apoptosis in normal CD34+38(-) cells. The effects against CML cells are seen when bortezomib is used alone and in combination with dasatinib. Bortezomib causes proteasome but not BCR-ABL inhibition and is also effective in inhibiting proteasome activity and inducing apoptosis in cell lines expressing BCR-ABL mutations, including T315I. By targeting both TKI-insensitive stem and progenitor cells and TKI-resistant BCR-ABL mutations, we believe that bortezomib offers a potential therapeutic option in CML. Because of known toxicities, including myelosuppression, the likely initial clinical application of bortezomib in CML would be in resistant and advanced disease.
Figures



 ), 10nM bortezomib (▩), and 20nM bortezomib (□). Cell recovery calculations for cells from all division peaks (E) and for undivided cells (F) in untreated and treated cells (bortezomib [bor] and dasatinib [das]). All results represent mean ± SEM with predicted dose-response curves.
), 10nM bortezomib (▩), and 20nM bortezomib (□). Cell recovery calculations for cells from all division peaks (E) and for undivided cells (F) in untreated and treated cells (bortezomib [bor] and dasatinib [das]). All results represent mean ± SEM with predicted dose-response curves.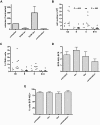

References
-
- Daley GQ, Van Etten RA, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990;247(4944):824–830. - PubMed
-
- Evans CA, Owen-Lynch PJ, Whetton AD, Dive C. Activation of the Abelson tyrosine kinase activity is associated with suppression of apoptosis in hemopoietic cells. Cancer Res. 1993;53(8):1735–1738. - PubMed
-
- Baccarani M, Saglio G, Goldman J, et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108(6):1809–1820. - PubMed
-
- Hochhaus A, O'Brien SG, Guilhot F, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009;23(6):1054–1061. - PubMed
-
- Druker BJ, Guilhot F, O'Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–2417. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

