Basal release of ATP: an autocrine-paracrine mechanism for cell regulation
- PMID: 20068232
- PMCID: PMC3085344
- DOI: 10.1126/scisignal.3104re1
Basal release of ATP: an autocrine-paracrine mechanism for cell regulation
Abstract
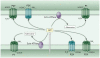
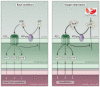
Cells release adenosine triphosphate (ATP), which activates plasma membrane-localized P2X and P2Y receptors and thereby modulates cellular function in an autocrine or paracrine manner. Release of ATP and the subsequent activation of P2 receptors help establish the basal level of activation (sometimes termed "the set point") for signal transduction pathways and regulate a wide array of responses that include tissue blood flow, ion transport, cell volume regulation, neuronal signaling, and host-pathogen interactions. Basal release and autocrine or paracrine responses to ATP are multifunctional, evolutionarily conserved, and provide an economical means for the modulation of cell, tissue, and organismal biology.
Figures
References
-
- Wollenberger A, Will H, Krause EG, Wollenberger A, Will H, Krause EG. Adenosine 3′,5′-monophosphate, the myocardial cell membrane, and calcium. Recent Adv. Stud. Cardiac Struct. Metab. 1975;5:81–93. - PubMed
-
- Tomkins GM. The metabolic code. Science. 1975;189:760–763. - PubMed
-
- Ménasché G, Kliche S, Bezman N, Schraven B. Regulation of T-cell antigen receptor-mediated inside-out signaling by cytosolic adapter proteins and Rap1 effector molecules. Immunol. Rev. 2007;218:82–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

