Nanoparticles targeted with NGR motif deliver c-myc siRNA and doxorubicin for anticancer therapy
- PMID: 20068551
- PMCID: PMC2862540
- DOI: 10.1038/mt.2009.291
Nanoparticles targeted with NGR motif deliver c-myc siRNA and doxorubicin for anticancer therapy
Abstract
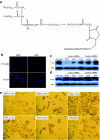
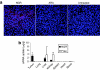
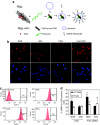
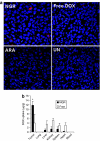
We have designed a PEGylated LPD (liposome-polycation-DNA) nanoparticle for systemic, specific, and efficient delivery of small interfering RNA (siRNA) into solid tumors in mice by modification with NGR (aspargine-glycine-arginine) peptide, targeting aminopeptidase N (CD13) expressed in the tumor cells or tumor vascular endothelium. LPD-PEG-NGR efficiently delivered siRNA to the cytoplasm and downregulated the target gene in the HT-1080 cells but not CD13(-) HT-29 cells, whereas nanoparticles containing a control peptide, LPD-PEG-ARA, showed only little siRNA uptake and gene silencing activity. LPD-PEG-NGR efficiently delivered siRNA into the cytoplasm of HT-1080 xenograft tumor 4 hours after intravenous injection. Three daily injections (1.2 mg/kg) of c-myc siRNA formulated in the LPD-PEG-NGR effectively suppressed c-myc expression and triggered cellular apoptosis in the tumor, resulting in a partial tumor growth inhibition. When doxorubicin (DOX) and siRNA were co-formulated in LPD-PEG-NGR, an enhanced therapeutic effect was observed.
Figures

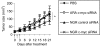
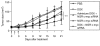
Similar articles
-
Multifunctional nanoparticles delivering small interfering RNA and doxorubicin overcome drug resistance in cancer.J Biol Chem. 2010 Jul 16;285(29):22639-50. doi: 10.1074/jbc.M110.125906. Epub 2010 May 11. J Biol Chem. 2010. PMID: 20460382 Free PMC article.
-
Co-delivery of CPP decorated doxorubicin and CPP decorated siRNA by NGR-modified nanobubbles for improving anticancer therapy.Pharm Dev Technol. 2021 Jul;26(6):634-646. doi: 10.1080/10837450.2021.1912090. Epub 2021 Apr 15. Pharm Dev Technol. 2021. PMID: 33843423
-
A self-assembled polyjuglanin nanoparticle loaded with doxorubicin and anti-Kras siRNA for attenuating multidrug resistance in human lung cancer.Biochem Biophys Res Commun. 2017 Dec 2;493(4):1430-1437. doi: 10.1016/j.bbrc.2017.09.132. Epub 2017 Sep 25. Biochem Biophys Res Commun. 2017. PMID: 28958938
-
Dual-modified liposomes with a two-photon-sensitive cell penetrating peptide and NGR ligand for siRNA targeting delivery.Biomaterials. 2015 Apr;48:84-96. doi: 10.1016/j.biomaterials.2015.01.030. Epub 2015 Feb 11. Biomaterials. 2015. PMID: 25701034
-
[Advance in studies on NGR peptide modified liposome and its anti-tumor performance].Zhongguo Zhong Yao Za Zhi. 2013 Jul;38(13):2041-5. Zhongguo Zhong Yao Za Zhi. 2013. PMID: 24079222 Review. Chinese.
Cited by
-
Application of Peptides in Construction of Nonviral Vectors for Gene Delivery.Nanomaterials (Basel). 2022 Nov 19;12(22):4076. doi: 10.3390/nano12224076. Nanomaterials (Basel). 2022. PMID: 36432361 Free PMC article. Review.
-
Pharmacokinetics and biodistribution of recently-developed siRNA nanomedicines.Adv Drug Deliv Rev. 2016 Sep 1;104:93-109. doi: 10.1016/j.addr.2015.12.004. Epub 2015 Dec 10. Adv Drug Deliv Rev. 2016. PMID: 26686832 Free PMC article. Review.
-
Nanoparticles as Drug Delivery Systems in Cancer Medicine: Emphasis on RNAi-Containing Nanoliposomes.Pharmaceuticals (Basel). 2013 Nov 4;6(11):1361-80. doi: 10.3390/ph6111361. Pharmaceuticals (Basel). 2013. PMID: 24287462 Free PMC article.
-
Codelivery of an optimal drug/siRNA combination using mesoporous silica nanoparticles to overcome drug resistance in breast cancer in vitro and in vivo.ACS Nano. 2013 Feb 26;7(2):994-1005. doi: 10.1021/nn3044066. Epub 2013 Jan 4. ACS Nano. 2013. PMID: 23289892 Free PMC article.
-
Targeting Integrins in Cancer Nanomedicine: Applications in Cancer Diagnosis and Therapy.Cancers (Basel). 2019 Nov 13;11(11):1783. doi: 10.3390/cancers11111783. Cancers (Basel). 2019. PMID: 31766201 Free PMC article. Review.
References
-
- Abaza MS, Al-Saffar A, Al-Sawan S., and , Al-Attiyah R. c-myc antisense oligonucleotides sensitize human colorectal cancer cells to chemotherapeutic drugs. Tumour Biol. 2008;29:287–303. - PubMed
-
- Pastorino F, Brignole C, Marimpietri D, Pagnan G, Morando A, Ribatti D, et al. Targeted liposomal c-myc antisense oligodeoxynucleotides induce apoptosis and inhibit tumor growth and metastases in human melanoma models. Clin Cancer Res. 2003;9:4595–4605. - PubMed
-
- Xia Z, Zhu Z, Zhang L, Royal C, Liu Z, Chen Q, et al. Specific reversal of MDR1/P-gp-dependent multidrug resistance by RNA interference in colon cancer cells. Oncol Rep. 2008;20:1433–1439. - PubMed
-
- Yadav S, van Vlerken LE, Little SR., and , Amiji MM. Evaluations of combination MDR-1 gene silencing and paclitaxel administration in biodegradable polymeric nanoparticle formulations to overcome multidrug resistance in cancer cells. Cancer Chemother Pharmacol. 2009;63:711–722. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

