Dendritic cells transduced with lentiviral vectors expressing VIP differentiate into VIP-secreting tolerogenic-like DCs
- PMID: 20068554
- PMCID: PMC2890107
- DOI: 10.1038/mt.2009.293
Dendritic cells transduced with lentiviral vectors expressing VIP differentiate into VIP-secreting tolerogenic-like DCs
Abstract
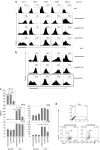
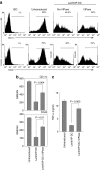
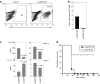
Dendritic cells (DCs) initiate immune responses as well as tolerance. We showed previously that the neuropeptide vasoactive intestinal peptide (VIP) suppresses innate immune responses, modulates adaptive responses by generating regulatory T cells (Treg) through the induction of tolerogenic DCs (tDCs), and has therapeutic effects in models of autoimmune/inflammatory disorders. Systemic VIP administration is limited by its short biological half-life and by its pleiotropic effects on the cardiovascular system and gastrointestinal tract. Therefore, we used lentiviral vectors to genetically engineer VIP-expressing bone marrow-derived DC (BMDC) and characterized the transduced LentiVIP-DC in terms of phenotype and therapeutic effects in models of experimental autoimmune encephalomyelitis (EAE) and cecal ligation and puncture (CLP) sepsis. LentiVIP-DCs secrete VIP, and resemble tDCs through lack of co-stimulatory molecule upregulation, lack of proinflammatory cytokine secretion, increased interleukin (IL)-10 production, and poor stimulation of allogeneic T cells. A single inoculation of LentiVIP-DC in EAE or CLP mice had therapeutic effects, which correlated with reduced expression of proinflammatory cytokines and increased IL-10 production in spinal cord and peritoneal fluid, respectively. In contrast to systemic VIP administration that requires repeated, high-dose inoculations, local delivery of VIP by LentiVIP-DC may represent a promising therapeutic tool for the treatment of autoimmune diseases and inflammatory disorders.
Figures




References
-
- Wolfraim LA. Treating autoimmune diseases through restoration of antigen-specific immune tolerance. Arch Immunol Ther Exp (Warsz) 2006;54:1–13. - PubMed
-
- Roth JC, Curiel DT., and , Pereboeva L. Cell vehicle targeting strategies. Gene Ther. 2008;15:716–729. - PubMed
-
- Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. - PubMed
-
- Banchereau J., and , Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

