Optimization of peptide nucleic acid antisense oligonucleotides for local and systemic dystrophin splice correction in the mdx mouse
- PMID: 20068555
- PMCID: PMC2848838
- DOI: 10.1038/mt.2009.310
Optimization of peptide nucleic acid antisense oligonucleotides for local and systemic dystrophin splice correction in the mdx mouse
Abstract
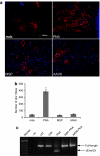
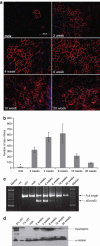
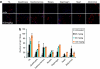
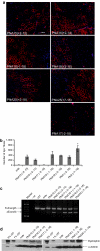
Antisense oligonucleotides (AOs) have the capacity to alter the processing of pre-mRNA transcripts in order to correct the function of aberrant disease-related genes. Duchenne muscular dystrophy (DMD) is a fatal X-linked muscle degenerative disease that arises from mutations in the DMD gene leading to an absence of dystrophin protein. AOs have been shown to restore the expression of functional dystrophin via splice correction by intramuscular and systemic delivery in animal models of DMD and in DMD patients via intramuscular administration. Major challenges in developing this splice correction therapy are to optimize AO chemistry and to develop more effective systemic AO delivery. Peptide nucleic acid (PNA) AOs are an alternative AO chemistry with favorable in vivo biochemical properties and splice correcting abilities. Here, we show long-term splice correction of the DMD gene in mdx mice following intramuscular PNA delivery and effective splice correction in aged mdx mice. Further, we report detailed optimization of systemic PNA delivery dose regimens and PNA AO lengths to yield splice correction, with 25-mer PNA AOs providing the greatest splice correcting efficacy, restoring dystrophin protein in multiple peripheral muscle groups. PNA AOs therefore provide an attractive candidate AO chemistry for DMD exon skipping therapy.
Figures
Similar articles
-
In vitro evaluation of novel antisense oligonucleotides is predictive of in vivo exon skipping activity for Duchenne muscular dystrophy.J Gene Med. 2010 Apr;12(4):354-64. doi: 10.1002/jgm.1446. J Gene Med. 2010. PMID: 20235089
-
Effective exon skipping and restoration of dystrophin expression by peptide nucleic acid antisense oligonucleotides in mdx mice.Mol Ther. 2008 Jan;16(1):38-45. doi: 10.1038/sj.mt.6300329. Epub 2007 Oct 30. Mol Ther. 2008. PMID: 17968354
-
Skipping multiple exons of dystrophin transcripts using cocktail antisense oligonucleotides.Nucleic Acid Ther. 2014 Feb;24(1):57-68. doi: 10.1089/nat.2013.0451. Epub 2013 Dec 31. Nucleic Acid Ther. 2014. PMID: 24380394 Review.
-
A fusion peptide directs enhanced systemic dystrophin exon skipping and functional restoration in dystrophin-deficient mdx mice.Hum Mol Genet. 2009 Nov 15;18(22):4405-14. doi: 10.1093/hmg/ddp395. Epub 2009 Aug 18. Hum Mol Genet. 2009. PMID: 19692354
-
Molecular correction of Duchenne muscular dystrophy by splice modulation and gene editing.RNA Biol. 2021 Jul;18(7):1048-1062. doi: 10.1080/15476286.2021.1874161. Epub 2021 Jan 20. RNA Biol. 2021. PMID: 33472516 Free PMC article. Review.
Cited by
-
Chemical approaches to discover the full potential of peptide nucleic acids in biomedical applications.Beilstein J Org Chem. 2021 Jul 19;17:1641-1688. doi: 10.3762/bjoc.17.116. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 34367346 Free PMC article. Review.
-
MOTS-c promotes phosphorodiamidate morpholino oligomer uptake and efficacy in dystrophic mice.EMBO Mol Med. 2021 Feb 5;13(2):e12993. doi: 10.15252/emmm.202012993. Epub 2020 Dec 18. EMBO Mol Med. 2021. PMID: 33337582 Free PMC article.
-
Antisense therapy in neurology.J Pers Med. 2013 Aug 2;3(3):144-76. doi: 10.3390/jpm3030144. J Pers Med. 2013. PMID: 25562650 Free PMC article. Review.
-
Targeting RNA splicing for disease therapy.Wiley Interdiscip Rev RNA. 2013 May-Jun;4(3):247-66. doi: 10.1002/wrna.1158. Epub 2013 Mar 19. Wiley Interdiscip Rev RNA. 2013. PMID: 23512601 Free PMC article. Review.
-
Using a State-of-the-Art Toolbox to Evaluate Molecular and Functional Readouts of Antisense Oligonucleotide-Induced Exon Skipping in mdx Mice.Nucleic Acid Ther. 2020 Feb;30(1):50-65. doi: 10.1089/nat.2019.0824. Epub 2019 Dec 10. Nucleic Acid Ther. 2020. PMID: 31821107 Free PMC article.
References
-
- Aartsma-Rus A, Kaman WE, Weij R, den Dunnen JT, van Ommen GJ., and , van Deutekom JC. Exploring the frontiers of therapeutic exon skipping for Duchenne muscular dystrophy by double targeting within one or multiple exons. Mol Ther. 2006;14:401–407. - PubMed
-
- Lu QL, Mann CJ, Lou F, Bou-Gharios G, Morris GE, Xue SA, et al. Functional amounts of dystrophin produced by skipping the mutated exon in the mdx dystrophic mouse. Nat Med. 2003;9:1009–1014. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

