A novel fragment derived from the beta chain of human fibrinogen, beta43-63, is a potent inhibitor of activated endothelial cells in vitro and in vivo
- PMID: 20068569
- PMCID: PMC2822935
- DOI: 10.1038/sj.bjc.6605495
A novel fragment derived from the beta chain of human fibrinogen, beta43-63, is a potent inhibitor of activated endothelial cells in vitro and in vivo
Abstract
Background: Angiogenesis and haemostasis are closely linked within tumours with many haemostatic proteins regulating tumour angiogenesis. Indeed we previously identified a fragment of human fibrinogen, fibrinogen E-fragment (FgnE) with potent anti-angiogenic properties in vitro and cytotoxic effects on tumour vessels in vivo. We therefore investigated which region of FgnE was mediating vessel cytotoxicity.
Methods: Human dermal microvascular endothelial cells (ECs) were used to test the efficacy of peptides derived from FgnE on proliferation, migration, differentiation, apoptosis and adhesion before testing the efficacy of an active peptide on tumour vasculature in vivo.
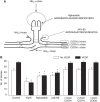
Results: We identified a 20-amino-acid peptide derived from the beta chain of FgnE, beta43-63, which had no effect on EC proliferation or migration but markedly inhibited the ability of activated ECs to form tubules or to adhere to various constituents of the extracellular matrix - collagen IV, fibronectin and vitronectin. Furthermore, our data show that beta43-63 interacts with ECs, in part, by binding to alpha(v)beta(3), so soluble alpha(v)beta(3) abrogated beta43-63 inhibition of tubule formation by activated ECs. Finally, when injected into mice bearing tumour xenografts, beta43-63 inhibited tumour vascularisation and induced formation of significant tumour necrosis.
Conclusions: Taken together, these data suggest that beta43-63 is a novel anti-tumour peptide whose anti-angiogenic effects are mediated by alpha(v)beta(3).
Figures

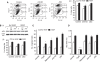
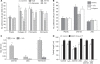

References
-
- Beckstead J (1994) A simple technique for preservation of fixation sensitive antigens in paraffin-embedded tissues. J Histochem Cytochem 42: 1127–1134 - PubMed
-
- Bootle-Wilbraham CA, Tazzyman S, Marshall JM, Lewis CE (2000) Fibrinogen E-fragment inhibits the migration and tubule formation of human dermal microvascular endothelial cells in vitro. Cancer Res 60: 4719–4724 - PubMed
-
- Borges E, Jan Y, Ruoslahti E (2000) Platelet derived growth factor receptor beta and vascular endothelial growth factor receptor 2 bind to the beta 3 integrin through its extracellular domain. J Biol Chem 275: 39867–39873 - PubMed
-
- Chua CC, Rahimi N, Forsten-Williams K, Nugent MA (2004) Heparan sulfate proteoglycans function as receptors for fibroblast growth factor-2 activation of extracellular signal-regulated kinases 1 and 2. Circ Res 94: 316–323 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

