Timing of appearance of late oligodendrocyte progenitors coincides with enhanced susceptibility of preterm rabbit cerebral white matter to hypoxia-ischemia
- PMID: 20068573
- PMCID: PMC2915781
- DOI: 10.1038/jcbfm.2009.286
Timing of appearance of late oligodendrocyte progenitors coincides with enhanced susceptibility of preterm rabbit cerebral white matter to hypoxia-ischemia
Abstract
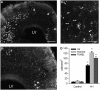
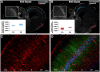
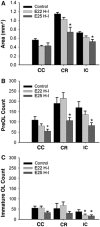
Emerging evidence supports that premature infants are susceptible to both cerebral white and gray matter injury. In a fetal rabbit model of placental insufficiency, preterm rabbits at embryonic day 22 (E22) exhibited histologic evidence of gray matter injury but minimal white matter injury after global hypoxia-ischemia (H-I). We hypothesized that the dissociation between susceptibility to gray and white matter injury at E22 was related to the timing of appearance of late oligodendrocyte progenitors (preOLs) that are particularly vulnerable in preterm human white matter lesions. During normal rabbit oligodendrocyte (OL) lineage progression, early OL progenitors predominated at E22. PreOL density increased between E24 and E25 in major forebrain white matter tracts. After H-I at E22 and E25, we observed a similar magnitude of cerebral H-I, assessed by cortical microvascular blood flow, and gray matter injury, assessed by caspase activation. However, the increased preOL density at E25 was accompanied by a significant increase in acute white matter injury after H-I that coincided with enhanced preOL degeneration. At E29, significant white matter atrophy developed after H-I at E25 but not E22. Thus, the timing of appearance of preOLs coincided with onset of a developmental window of enhanced white but not gray matter susceptibility to H-I.
Figures
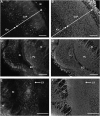
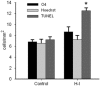
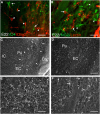
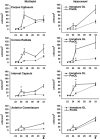
Similar articles
-
Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury.Ann Neurol. 2008 Apr;63(4):520-30. doi: 10.1002/ana.21359. Ann Neurol. 2008. PMID: 18393269 Free PMC article.
-
Strain-specific differences in perinatal rodent oligodendrocyte lineage progression and its correlation with human.Dev Neurosci. 2011;33(3-4):251-60. doi: 10.1159/000327242. Epub 2011 Aug 25. Dev Neurosci. 2011. PMID: 21865655 Free PMC article.
-
White matter injury in the preterm infant: pathology and mechanisms.Acta Neuropathol. 2017 Sep;134(3):331-349. doi: 10.1007/s00401-017-1718-6. Epub 2017 May 22. Acta Neuropathol. 2017. PMID: 28534077 Free PMC article. Review.
-
Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia.J Neurosci. 2002 Jan 15;22(2):455-63. doi: 10.1523/JNEUROSCI.22-02-00455.2002. J Neurosci. 2002. PMID: 11784790 Free PMC article.
-
Role of instrumented fetal sheep preparations in defining the pathogenesis of human periventricular white-matter injury.J Child Neurol. 2006 Jul;21(7):582-9. doi: 10.1177/08830738060210070101. J Child Neurol. 2006. PMID: 16970848 Review.
Cited by
-
Fetal Growth Restriction Impairs Lung Function and Neurodevelopment in an Early Preterm Rabbit Model.Biomedicines. 2023 Jan 5;11(1):139. doi: 10.3390/biomedicines11010139. Biomedicines. 2023. PMID: 36672647 Free PMC article.
-
Tetrahydrobiopterin in Cell Function and Death Mechanisms.Antioxid Redox Signal. 2022 Jul;37(1-3):171-183. doi: 10.1089/ars.2021.0136. Epub 2022 Jan 27. Antioxid Redox Signal. 2022. PMID: 34806400 Free PMC article. Review.
-
Enhanced nociceptive behavior and expansion of associated primary afferents in a rabbit model of cerebral palsy.J Neurosci Res. 2022 Oct;100(10):1951-1966. doi: 10.1002/jnr.25108. Epub 2022 Jul 15. J Neurosci Res. 2022. PMID: 35839339 Free PMC article.
-
Spinal cord injury in hypertonic newborns after antenatal hypoxia-ischemia in a rabbit model of cerebral palsy.Exp Neurol. 2017 Jul;293:13-26. doi: 10.1016/j.expneurol.2017.03.017. Epub 2017 Mar 24. Exp Neurol. 2017. PMID: 28347765 Free PMC article.
-
Hypoxia-ischemia is not an antecedent of most preterm brain damage: the illusion of validity.Dev Med Child Neurol. 2018 Feb;60(2):120-125. doi: 10.1111/dmcn.13483. Epub 2017 Jun 28. Dev Med Child Neurol. 2018. PMID: 28656697 Free PMC article. Review.
References
-
- Back S, Craig A, Luo N, Ren J, Akundi R, Rebeiro I, Rivkees S. Protective effects of caffeine on chronichypoxia-induced perinatal white matter injury. Ann Neurol. 2006;60:696–705. - PubMed
-
- Back SA. Perinatal white matter injury: the changing spectrum of pathologyand emerging insights into pathogenetic mechanisms. MRDD Res Rev. 2006;12:129–140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

