Isoflurane anesthesia induced persistent, progressive memory impairment, caused a loss of neural stem cells, and reduced neurogenesis in young, but not adult, rodents
- PMID: 20068576
- PMCID: PMC2949194
- DOI: 10.1038/jcbfm.2009.274
Isoflurane anesthesia induced persistent, progressive memory impairment, caused a loss of neural stem cells, and reduced neurogenesis in young, but not adult, rodents
Abstract
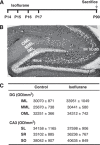
Isoflurane and related anesthetics are widely used to anesthetize children, ranging from premature babies to adolescents. Concerns have been raised about the safety of these anesthetics in pediatric patients, particularly regarding possible negative effects on cognition. The purpose of this study was to investigate the effects of repeated isoflurane exposure of juvenile and mature animals on cognition and neurogenesis. Postnatal day 14 (P14) rats and mice, as well as adult (P60) rats, were anesthetized with isoflurane for 35 mins daily for four successive days. Object recognition, place learning and reversal learning as well as cell death and cytogenesis were evaluated. Object recognition and reversal learning were significantly impaired in isoflurane-treated young rats and mice, whereas adult animals were unaffected, and these deficits became more pronounced as the animals grew older. The memory deficit was paralleled by a decrease in the hippocampal stem cell pool and persistently reduced neurogenesis, subsequently causing a reduction in the number of dentate gyrus granule cell neurons in isoflurane-treated rats. There were no signs of increased cell death of progenitors or neurons in the hippocampus. These findings show a previously unknown mechanism of neurotoxicity, causing cognitive deficits in a clearly age-dependent manner.
Figures
 treatment × time.
treatment × time.  P<0.05, **/##P<0.01, ***/###P<0.001.)
P<0.05, **/##P<0.01, ***/###P<0.001.)

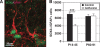
Similar articles
-
Effects of isoflurane or propofol on postnatal hippocampal neurogenesis in young and aged rats.Brain Res. 2013 Sep 12;1530:1-12. doi: 10.1016/j.brainres.2013.07.035. Epub 2013 Jul 24. Brain Res. 2013. PMID: 23891717
-
Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats.Anesthesiology. 2009 Apr;110(4):834-48. doi: 10.1097/ALN.0b013e31819c463d. Anesthesiology. 2009. PMID: 19293705
-
Isoflurane does not affect brain cell death, hippocampal neurogenesis, or long-term neurocognitive outcome in aged rats.Anesthesiology. 2010 Feb;112(2):305-15. doi: 10.1097/ALN.0b013e3181ca33a1. Anesthesiology. 2010. PMID: 20098132 Free PMC article.
-
Beyond anesthetic properties: the effects of isoflurane on brain cell death, neurogenesis, and long-term neurocognitive function.Anesth Analg. 2010 Feb;110(2):431-7. doi: 10.1213/ANE.0b013e3181af8015. Anesth Analg. 2010. PMID: 19917621 Review.
-
Beyond anesthetic properties: the effects of isoflurane on brain cell death, neurogenesis, and long-term neurocognitive function.Anesth Analg. 2010 Feb;110(2):431-7. Anesth Analg. 2010. PMID: 25508825 Review.
Cited by
-
Behavioral and emotional effects of repeated general anesthesia in young children.Saudi J Anaesth. 2015 Apr-Jun;9(2):161-6. doi: 10.4103/1658-354X.152843. Saudi J Anaesth. 2015. PMID: 25829904 Free PMC article.
-
Long-term effects of neonatal single or multiple isoflurane exposures on spatial memory in rats.Front Neurol. 2013 Jul 8;4:87. doi: 10.3389/fneur.2013.00087. eCollection 2013. Front Neurol. 2013. PMID: 23847588 Free PMC article.
-
Impaired KDM2B-mediated PRC1 recruitment to chromatin causes defective neural stem cell self-renewal and ASD/ID-like behaviors.iScience. 2022 Jan 7;25(2):103742. doi: 10.1016/j.isci.2022.103742. eCollection 2022 Feb 18. iScience. 2022. PMID: 35128353 Free PMC article.
-
Intergenerational Effects of Sevoflurane in Young Adult Rats.Anesthesiology. 2019 Nov;131(5):1092-1109. doi: 10.1097/ALN.0000000000002920. Anesthesiology. 2019. PMID: 31517640 Free PMC article.
-
A double-edged sword: volatile anesthetic effects on the neonatal brain.Brain Sci. 2014 Apr 16;4(2):273-94. doi: 10.3390/brainsci4020273. Brain Sci. 2014. PMID: 24961761 Free PMC article.
References
-
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. - PubMed
-
- Altman J, Bayer SA. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol. 1990;301:365–381. - PubMed
-
- Bertaina-Anglade V, Enjuanes E, Morillon D, Drieu la Rochelle C. The object recognition task in rats and mice: a simple and rapid model in safety pharmacology to detect amnesic properties of a new chemical entity. J Pharmacol Toxicol Methods. 2006;54:99–105. - PubMed
-
- Culley DJ, Baxter MG, Yukhananov R, Crosby G. impairment of acquisition of a spatial memory task followingisoflurane-nitrous oxide anesthesia in rats. Anesthesiology. 2004;100:309–314. - PubMed
-
- Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, Holtas S, van Roon-Mom WM, Bjork-Eriksson T, Nordborg C, Frisen J, Dragunow M, Faull RL, Eriksson PS. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science (NY) 2007;315:1243–1249. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

