STEMI in a 24-year-old man after use of a synephrine-containing dietary supplement: a case report and review of the literature
- PMID: 20069086
- PMCID: PMC2801940
STEMI in a 24-year-old man after use of a synephrine-containing dietary supplement: a case report and review of the literature
Abstract
Billions of dollars are spent annually in the United States in the largely unregulated market of dietary supplements. Many of these supplements are marketed as weight-loss and athletic-performance-enhancement products. The association of various ephedra-containing products with adverse cardiovascular events has led to a ban on the sale of these products by the U.S. Food and Drug Administration. The result has been the emergence of new formulations marketed for weight loss and athletic-performance enhancement that are "ephedra-free" but contain other sympathomimetic substances, the safety of which has not been established. We present the case of a previously healthy 24-year-old man who presented with an ST-segment-elevation myocardial infarction (STEMI) within hours of taking the ephedra-free product Nutrex Lipo-6x. Emergent coronary angiography revealed the presence of extensive, diffuse thrombus in the left anterior descending coronary artery. The patient had no risk factors for coronary artery disease or myocardial infarction; this includes the absence of a hypercoagulable state and the absence of a history of illicit drug use. This case of STEMI--associated as it is with the use of a synephrine-containing product by a person without risk factors for coronary artery disease--is to our knowledge the 1st reported in the literature. We discuss the patient's evaluation and clinical course, and we review the literature with respect to synephrine-containing dietary supplements. On the basis of synephrine's chemical composition and mechanism of action, we propose a direct association between this patient's use of Nutrex Lipo-6x and his STEMI.
Keywords: Citrus/adverse effects; United States Food and Drug Administration; coronary vasospasm/complications/etiology; dietary supplements/adverse effects/poisoning; myocardial infarction/chemically induced; plant preparations; synephrine; weight loss/drug effects.
Figures

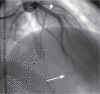

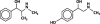
Comment in
-
Lack of evidence that p-synephrine is responsible for STEMI.Tex Heart Inst J. 2010;37(3):383; author reply 383-4. Tex Heart Inst J. 2010. PMID: 20548833 Free PMC article. No abstract available.
References
-
- Haller CA, Benowitz NL. Adverse cardiovascular and central nervous system events associated with dietary supplements containing ephedra alkaloids. N Engl J Med 2000;343(25): 1833–8. - PubMed
-
- Samenuk D, Link MS, Homoud MK, Contreras R, Theoharides TC, Wang PJ, Estes NA 3rd. Adverse cardiovascular events temporally associated with ma huang, an herbal source of ephedrine. Mayo Clin Proc 2002;77(1):12–6. - PubMed
-
- Sales of Supplements Containing Ephedrine Alkaloids (Ephedra) Prohibited [cited 2009 Oct 13]. Available from: http://www.fda.gov/Food/DietarySupplements/GuidanceComplianceRegulatoryI...
-
- Vincent GM, Anderson JL, Marshall HW. Coronary spasm producing coronary thrombosis and myocardial infarction. N Engl J Med 1983;309(4):220–3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
