Reduction of monocyte chemoattractant protein-1 and interleukin-8 levels by ticlopidine in TNF-alpha stimulated human umbilical vein endothelial cells
- PMID: 20069129
- PMCID: PMC2804117
- DOI: 10.1155/2009/917837
Reduction of monocyte chemoattractant protein-1 and interleukin-8 levels by ticlopidine in TNF-alpha stimulated human umbilical vein endothelial cells
Abstract
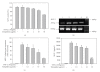
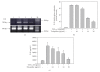
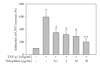
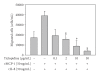
Atherosclerosis and its associated complications represent major causes of morbidity and mortality in the industrialized or Western countries. Monocyte chemoattractant protein-1 (MCP-1) is critical for the initiating and developing of atherosclerotic lesions. Interleukin-8 (IL-8), a CXC chemokine, stimulates neutrophil chemotaxis. Ticlopidine is one of the antiplatelet drugs used to prevent thrombus formation relevant to the pathophysiology of atherothrombosis. In this study, we found that ticlopidine dose-dependently decreased the mRNA and protein levels of TNF-alpha-stimulated MCP-1, IL-8, and vascular cell adhesion molecule-1 (VCAM-1) in human umbilical vein endothelial cells (HUVECs). Ticlopidine declined U937 cells adhesion and chemotaxis as compared to TNF-alpha stimulated alone. Furthermore, the inhibitory effects were neither due to decreased HUVEC viability, nor through NF-kB inhibition. These results suggest that ticlopidine decreased TNF-alpha induced MCP-1, IL-8, and VCAM-1 levels in HUVECs, and monocyte adhesion. Therefore, the data provide additional therapeutic machinery of ticlopidine in treatment and prevention of atherosclerosis.
Figures

Similar articles
-
Aspirin inhibits monocyte chemoattractant protein-1 and interleukin-8 expression in TNF-alpha stimulated human umbilical vein endothelial cells.Atherosclerosis. 2004 Jun;174(2):207-13. doi: 10.1016/j.atherosclerosis.2004.01.024. Atherosclerosis. 2004. PMID: 15136050
-
Dehydroepiandrosterone inhibits the TNF-alpha-induced inflammatory response in human umbilical vein endothelial cells.Atherosclerosis. 2007 Jan;190(1):90-9. doi: 10.1016/j.atherosclerosis.2006.02.031. Epub 2006 Mar 30. Atherosclerosis. 2007. PMID: 16574124
-
Effect of caffeic acid on tumor necrosis factor-alpha-induced vascular inflammation in human umbilical vein endothelial cells.Biol Pharm Bull. 2009 Aug;32(8):1371-7. doi: 10.1248/bpb.32.1371. Biol Pharm Bull. 2009. PMID: 19652376
-
Protocatechuic aldehyde suppresses TNF-alpha-induced ICAM-1 and VCAM-1 expression in human umbilical vein endothelial cells.Eur J Pharmacol. 2005 Apr 18;513(1-2):1-8. doi: 10.1016/j.ejphar.2005.01.059. Eur J Pharmacol. 2005. PMID: 15878704
-
Chloroform extract of aged black garlic attenuates TNF-α-induced ROS generation, VCAM-1 expression, NF-κB activation and adhesiveness for monocytes in human umbilical vein endothelial cells.Phytother Res. 2011 Jan;25(1):92-100. doi: 10.1002/ptr.3230. Phytother Res. 2011. PMID: 20623600
Cited by
-
The Protective Effects of a Synthetic Geranyl Acetophenone in a Cellular Model of TNF-α-Induced Pulmonary Epithelial Barrier Dysfunction.Molecules. 2018 Jun 5;23(6):1355. doi: 10.3390/molecules23061355. Molecules. 2018. PMID: 29874809 Free PMC article.
-
Low-dose TNF augments fracture healing in normal and osteoporotic bone by up-regulating the innate immune response.EMBO Mol Med. 2015 May;7(5):547-61. doi: 10.15252/emmm.201404487. EMBO Mol Med. 2015. PMID: 25770819 Free PMC article.
-
ROS-induced ZNF580 expression: a key role for H2O2/NF-κB signaling pathway in vascular endothelial inflammation.Mol Cell Biochem. 2012 Jan;359(1-2):183-91. doi: 10.1007/s11010-011-1013-0. Epub 2011 Aug 10. Mol Cell Biochem. 2012. PMID: 21830064
References
-
- Terkeltaub R, Boisvert WA, Curtiss LK. Chemokines and atherosclerosis. Current Opinion in Lipidology. 1998;9(5):397–405. - PubMed
-
- Reape TJ, Groot PHE. Chemokines and atherosclerosis. Atherosclerosis. 1999;147(2):213–225. - PubMed
-
- Dosquet C, Weill D, Wautier JL. Cytokines and thrombosis. Journal of Cardiovascular Pharmacology. 1995;25(supplement 2):S13–S19. - PubMed
-
- Braunersreuther V, Mach F, Steffens S. The specific role of chemokines in atherosclerosis. Thrombosis and Haemostasis. 2007;97(5):714–721. - PubMed
-
- Navab M, Imes SS, Hama SY, et al. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. Journal of Clinical Investigation. 1991;88(6):2039–2046. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

