Functional alterations in memory networks in early Alzheimer's disease
- PMID: 20069392
- PMCID: PMC3036844
- DOI: 10.1007/s12017-009-8109-7
Functional alterations in memory networks in early Alzheimer's disease
Abstract
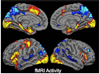
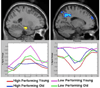
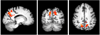
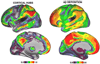
The hallmark clinical symptom of early Alzheimer's disease (AD) is episodic memory impairment. Recent functional imaging studies suggest that memory function is subserved by a set of distributed networks, which include both the medial temporal lobe (MTL) system and the set of cortical regions collectively referred to as the default network. Specific regions of the default network, in particular, the posteromedial cortices, including the precuneus and posterior cingulate, are selectively vulnerable to early amyloid deposition in AD. These regions are also thought to play a key role in both memory encoding and retrieval, and are strongly functionally connected to the MTL. Multiple functional magnetic resonance imaging (fMRI) studies during memory tasks have revealed alterations in these networks in patients with clinical AD. Similar functional abnormalities have been detected in subjects at-risk for AD, including those with genetic risk and older individuals with mild cognitive impairment. Recently, we and other groups have found evidence of functional alterations in these memory networks even among cognitively intact older individuals with occult amyloid pathology, detected by PET amyloid imaging. Taken together, these findings suggest that the pathophysiological process of AD exerts specific deleterious effects on these distributed memory circuits, even prior to clinical manifestations of significant memory impairment. Interestingly, some of the functional alterations seen in prodromal AD subjects have taken the form of increases in activity relative to baseline, rather than a loss of activity. It remains unclear whether these increases in fMRI activity may be compensatory to maintain memory performance in the setting of early AD pathology or instead, represent evidence of excitotoxicity and impending neuronal failure. Recent studies have also revealed disruption of the intrinsic connectivity of these networks observable even during the resting state in early AD and asymptomatic individuals with high amyloid burden. Research is ongoing to determine if these early network alterations will serve as sensitive predictors of clinical decline, and eventually, as markers of pharmacological response to potential disease-modifying treatments for AD.
Figures

References
-
- Alexander GE, Chen K, Pietrini P, Rapoport SI, Reiman EM. Longitudinal PET evaluation of cerebral metabolic decline in dementia: A potential outcome measure in Alzheimer’s disease treatment studies. American Journal of Psychiatry. 2002;159:738–745. - PubMed
-
- Alpar A, Ueberham U, Bruckner MK, Seeger G, Arendt T, Gartner U. Different dendrite and dendritic spine alterations in basal and apical arbors in mutant human amyloid precursor protein transgenic mice. Brain Research. 2006;1099(1):189–198. - PubMed
-
- Amieva H, Le Goff M, Millet X, Orgogozo JM, Peres K, Barberger-Gateau P, et al. Prodromal Alzheimer’s disease: Successive emergence of the clinical symptoms. Annals of Neurology. 2008;64:492–498. - PubMed
-
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cerebral Cortex. 1991;1:103–116. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

