Mass spectrometric evidence for the existence of distinct modifications of different proteins by 2(E),4(E)-decadienal
- PMID: 20070074
- PMCID: PMC2838956
- DOI: 10.1021/tx900379a
Mass spectrometric evidence for the existence of distinct modifications of different proteins by 2(E),4(E)-decadienal
Abstract
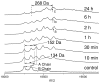
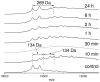
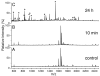
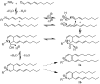
2(E),4(E)-Decadienal (DDE), a lipid peroxidation product, was found to covalently modify Lys residues of different proteins by different reactions using mass spectrometry (MALDI-TOF-MS and LC-ESI-MS). DDE mainly formed Lys Schiff base adducts with cytochrome c and ribonuclease A at 10 min, but these reversibly formed adducts almost disappeared after 24 h. In contrast, beta-lactoglobulin (beta-LG) was highly modified by DDE after 24 h. In addition to the Lys Schiff base adducts, DDE formed novel Lys pyridinium adducts as well as Cys Michael adducts with beta-LG.
Figures
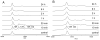
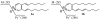

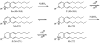
References
-
- Szweda LI, Uchida K, Tsai L, Stadtman ER. Inactivation of glucose-6-phosphate dehydrogenase by 4-hydroxy-2-nonenal. Selective modification of an active-site lysine. J Biol Chem. 1993;268:3342–3347. - PubMed
-
- Nadkarni DV, Sayre LM. Structural definition of early lysine and histidine adduction chemistry of 4-hydroxynonenal. Chem Res Toxicol. 1995;8:284–291. - PubMed
-
- Doorn JA, Petersen DR. Covalent modification of amino acid nucleophiles by the lipid peroxidation products 4-hydroxy-2-nonenal and 4-oxo-2-nonenal. Chem Res Toxicol. 2002;15:1445–1450. - PubMed
-
- Liu Z, Minkler PE, Sayre LM. Mass spectroscopic characterization of protein modification by 4-hydroxy-2-(E)-nonenal and 4-oxo-2-(E)-nonenal. Chem Res Toxicol. 2003;16:901–911. - PubMed
-
- Cohn JA, Tsai L, Friguet B, Szweda LI. Chemical characterization of a protein-4-hydroxy-2-nonenal cross-link: immunochemical detection in mitochondria exposed to oxidative stress. Arch Biochem Biophys. 1996;328:158–164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

