Ultrastructural features of retinal capillary basement membrane thickening in diabetic swine
- PMID: 20070152
- PMCID: PMC3085508
- DOI: 10.3109/01913120903308583
Ultrastructural features of retinal capillary basement membrane thickening in diabetic swine
Abstract
Purpose: To assess retinal capillary basement membrane thickening (BMT) in a swine model of type 1 diabetes.
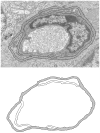
Materials and methods: Yorkshire pigs were rendered diabetic with streptozotocin and dyslipidemic with a high fat and cholesterol diet. At 18, 26, and 32 weeks of diabetes, the retina sections within 3 disc diameters from the optic disc were examined under transmission electron microscopy to evaluate the ultrastructural features of the capillary BM. Digital morphometric analysis was performed to measure BMT.
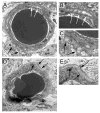
Results: Diabetic swine had significantly thicker retinal capillary BMs compared to controls. Pigs that sustained diabetes for longer periods or experienced severe diabetes tended to have more BMT. Those pigs that did not sustain glucose levels above 200 mg/dL did not demonstrate thicker retinal capillary BMs. Characteristic ultrastructural features of diabetic vasculopathy observed included rarefaction as an early stage of Swiss cheese cavitation, lamellation with multiplication of electron dense layers, and fibrillar materials within capillary BM.
Conclusions: Diabetic Yorkshire pigs develop characteristic features of an early retinal microvasculopathy fairly rapidly and may serve as a higher-order animal model for studies of type 1 diabetes.
Figures


References
-
- Frank RN. Diabetic retinopathy. N Engl J Med. 2004;350:48–58. - PubMed
-
- Bloodworth JM., Jr Diabetic microangiopathy. Diabetes. 1963;12:99–114. - PubMed
-
- Robison WG, Jr, Kador PF, Kinoshita JH. Retinal capillaries: basement membrane thickening by galactosemia prevented with aldose reductase inhibitor. Science. 1983;221:1177–1179. - PubMed
-
- Toussaint D, Dustin P. Electron microscopy of normal and diabetic retinal capillaries. Arch Ophthalmol. 1963;70:96–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
