Developmental reprogramming of reproductive and metabolic dysfunction in sheep: native steroids vs. environmental steroid receptor modulators
- PMID: 20070410
- PMCID: PMC3970726
- DOI: 10.1111/j.1365-2605.2009.01024.x
Developmental reprogramming of reproductive and metabolic dysfunction in sheep: native steroids vs. environmental steroid receptor modulators
Abstract
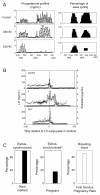
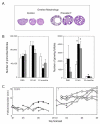
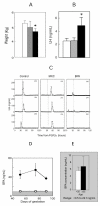
The inappropriate programming of developing organ systems by exposure to excess native or environmental steroids, particularly the contamination of our environment and our food sources with synthetic endocrine disrupting chemicals that can interact with steroid receptors, is a major concern. Studies with native steroids have found that in utero exposure of sheep to excess testosterone, an oestrogen precursor, results in low birth weight offspring and leads to an array of adult reproductive/metabolic deficits manifested as cycle defects, functional hyperandrogenism, neuroendocrine/ovarian defects, insulin resistance and hypertension. Furthermore, the severity of reproductive dysfunction is amplified by excess postnatal weight gain. The constellation of adult reproductive and metabolic dysfunction in prenatal testosterone-treated sheep is similar to features seen in women with polycystic ovary syndrome. Prenatal dihydrotestosterone treatment failed to result in similar phenotype suggesting that many effects of prenatal testosterone excess are likely facilitated via aromatization to oestradiol. Similarly, exposure to environmental steroid imposters such as bisphenol A (BPA) and methoxychlor (MXC) from days 30 to 90 of gestation had long-term but differential effects. Exposure of sheep to BPA, which resulted in maternal levels of 30-50 ng/mL BPA, culminated in low birth weight offspring. These female offspring were hypergonadotropic during early postnatal life and characterized by severely dampened preovulatory LH surges. Prenatal MXC-treated females had normal birth weight and manifested delayed but normal amplitude LH surges. Importantly, the effects of BPA were evident at levels, which approximated twice the highest levels found in human maternal circulation of industrialized nations. These findings provide evidence in support of developmental origin of adult reproductive and metabolic diseases and highlight the risk posed by exposure to environmental endocrine disrupting chemicals.
Figures

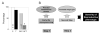

Similar articles
-
Developmental programming: differential effects of prenatal exposure to bisphenol-A or methoxychlor on reproductive function.Endocrinology. 2006 Dec;147(12):5956-66. doi: 10.1210/en.2006-0805. Epub 2006 Aug 31. Endocrinology. 2006. PMID: 16946013
-
Reproduction Symposium: developmental programming of reproductive and metabolic health.J Anim Sci. 2014 Aug;92(8):3199-210. doi: 10.2527/jas.2014-7637. J Anim Sci. 2014. PMID: 25074449 Free PMC article. Review.
-
Developmental programming: impact of prenatal exposure to bisphenol-A and methoxychlor on steroid feedbacks in sheep.Toxicol Appl Pharmacol. 2013 May 1;268(3):300-8. doi: 10.1016/j.taap.2013.02.011. Epub 2013 Feb 27. Toxicol Appl Pharmacol. 2013. PMID: 23454450 Free PMC article.
-
Steroidogenic versus Metabolic Programming of Reproductive Neuroendocrine, Ovarian and Metabolic Dysfunctions.Neuroendocrinology. 2015;102(3):226-37. doi: 10.1159/000381830. Epub 2015 Apr 1. Neuroendocrinology. 2015. PMID: 25832114 Free PMC article. Review.
-
Developmental programming: impact of fetal exposure to endocrine-disrupting chemicals on gonadotropin-releasing hormone and estrogen receptor mRNA in sheep hypothalamus.Toxicol Appl Pharmacol. 2010 Sep 1;247(2):98-104. doi: 10.1016/j.taap.2010.05.017. Epub 2010 Jun 4. Toxicol Appl Pharmacol. 2010. PMID: 20621667 Free PMC article.
Cited by
-
Sheep models of polycystic ovary syndrome phenotype.Mol Cell Endocrinol. 2013 Jul 5;373(1-2):8-20. doi: 10.1016/j.mce.2012.10.005. Epub 2012 Oct 16. Mol Cell Endocrinol. 2013. PMID: 23084976 Free PMC article. Review.
-
Developmental programming: Adipose depot-specific changes and thermogenic adipocyte distribution in the female sheep.Mol Cell Endocrinol. 2020 Mar 1;503:110691. doi: 10.1016/j.mce.2019.110691. Epub 2019 Dec 19. Mol Cell Endocrinol. 2020. PMID: 31863810 Free PMC article.
-
Developmental programming: Adipose depot-specific regulation of non-coding RNAs and their relation to coding RNA expression in prenatal testosterone and prenatal bisphenol-A -treated female sheep.Mol Cell Endocrinol. 2023 Mar 15;564:111868. doi: 10.1016/j.mce.2023.111868. Epub 2023 Jan 26. Mol Cell Endocrinol. 2023. PMID: 36708980 Free PMC article.
-
Developmental programming: Sex-specific programming of growth upon prenatal bisphenol A exposure.J Appl Toxicol. 2019 Nov;39(11):1516-1531. doi: 10.1002/jat.3836. Epub 2019 Jul 23. J Appl Toxicol. 2019. PMID: 31338854 Free PMC article.
-
Developmental programming: Impact of prenatal testosterone treatment and postnatal obesity on ovarian follicular dynamics.J Dev Orig Health Dis. 2012 Aug;3(4):276-86. doi: 10.1017/S2040174412000128. J Dev Orig Health Dis. 2012. PMID: 23766891 Free PMC article.
References
-
- Abbott DH, Dumesic DA, Levine JE, Dunaif A, Padmanabhan V. Animal models and fetal programming of PCOS. In: Azziz R, Nestler JE, Dewailly D, editors. Contemporary Endocrinology: Androgen Excess Disorders in Women: Polycystic Ovary Syndrome and Other Disorders. Humana Press Inc; Totowa, New Jersey: 2006. pp. 259–272.
-
- Barker DJP. Mothers, babies, and disease in later life. BMJ Publishing Group; 1994. Programming the baby; pp. 14–36.
-
- Birch RA, Padmanabhan V, Foster DL, Robinson JE. Prenatal programming of reproductive neuroendocrine function: fetal androgen exposure produces progressive disruption of reproductive cycles in sheep. Endocrinology. 2003;144:1426–1434. - PubMed
-
- Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290–296. - PubMed

