Tendon and ligament fibrillar crimps give rise to left-handed helices of collagen fibrils in both planar and helical crimps
- PMID: 20070421
- PMCID: PMC2829388
- DOI: 10.1111/j.1469-7580.2009.01188.x
Tendon and ligament fibrillar crimps give rise to left-handed helices of collagen fibrils in both planar and helical crimps
Abstract
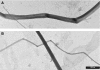
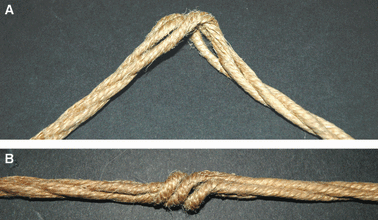
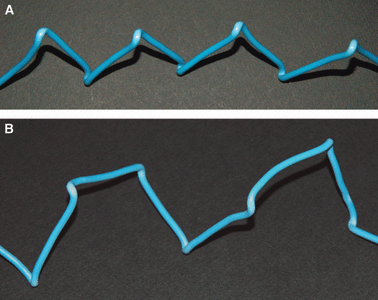
Collagen fibres in tendons and ligaments run straight but in some regions they show crimps which disappear or appear more flattened during the initial elongation of tissues. Each crimp is formed of collagen fibrils showing knots or fibrillar crimps at the crimp top angle. The present study analyzes by polarized light microscopy, scanning electron microscopy, transmission electron microscopy the 3D morphology of fibrillar crimp in tendons and ligaments of rat demonstrating that each fibril in the fibrillar region always twists leftwards changing the plane of running and sharply bends modifying the course on a new plane. The morphology of fibrillar crimp in stretched tendons fulfills the mechanical role of the fibrillar crimp acting as a particular knot/biological hinge in absorbing tension forces during fibril strengthening and recoiling collagen fibres when stretching is removed. The left-handed path of fibrils in the fibrillar crimp region gives rise to left-handed fibril helices observed both in isolated fibrils and sections of different tendons and ligaments (flexor digitorum profundus muscle tendon, Achilles tendon, tail tendon, patellar ligament and medial collateral ligament of the knee). The left-handed path of fibrils represents a new final suprafibrillar level of the alternating handedness which was previously described only from the molecular to the microfibrillar level. When the width of the twisting angle in the fibrillar crimp is nearly 180 degrees the fibrils appear as left-handed flattened helices forming crimped collagen fibres previously described as planar crimps. When fibrils twist with different subsequent rotational angles (< 180 degrees ) they always assume a left-helical course but, running in many different nonplanar planes, they form wider helical crimped fibres.
Figures









Similar articles
-
Contribution of glycosaminoglycans to the microstructural integrity of fibrillar and fiber crimps in tendons and ligaments.ScientificWorldJournal. 2010 Oct 1;10:1932-40. doi: 10.1100/tsw.2010.192. ScientificWorldJournal. 2010. PMID: 20890582 Free PMC article.
-
Crimp morphology in relaxed and stretched rat Achilles tendon.J Anat. 2007 Jan;210(1):1-7. doi: 10.1111/j.1469-7580.2006.00666.x. J Anat. 2007. PMID: 17229278 Free PMC article.
-
Tendon crimps and peritendinous tissues responding to tensional forces.Eur J Histochem. 2007;51 Suppl 1:9-14. Eur J Histochem. 2007. PMID: 17703588
-
Collagen structure of tendon relates to function.ScientificWorldJournal. 2007 Mar 30;7:404-20. doi: 10.1100/tsw.2007.92. ScientificWorldJournal. 2007. PMID: 17450305 Free PMC article. Review.
-
Hierarchical structures in fibrillar collagens.Micron. 2002;33(7-8):587-96. doi: 10.1016/s0968-4328(02)00033-1. Micron. 2002. PMID: 12475555 Review.
Cited by
-
The Specific Molecular Composition and Structural Arrangement of Eleutherodactylus Coqui Gular Skin Tissue Provide Its High Mechanical Compliance.Int J Mol Sci. 2020 Aug 5;21(16):5593. doi: 10.3390/ijms21165593. Int J Mol Sci. 2020. PMID: 32764252 Free PMC article.
-
Imaging the Nonlinear Susceptibility Tensor of Collagen by Nonlinear Optical Stokes Ellipsometry.Biophys J. 2016 Oct 4;111(7):1361-1374. doi: 10.1016/j.bpj.2016.05.055. Biophys J. 2016. PMID: 27705760 Free PMC article.
-
Optical Microscopy and Electron Microscopy for the Morphological Evaluation of Tendons: A Mini Review.Orthop Surg. 2020 Apr;12(2):366-371. doi: 10.1111/os.12637. Epub 2020 Feb 25. Orthop Surg. 2020. PMID: 32096911 Free PMC article. Review.
-
Ring-Mesh Model of Proteoglycan Glycosaminoglycan Chains in Tendon based on Three-dimensional Reconstruction by Focused Ion Beam Scanning Electron Microscopy.J Biol Chem. 2016 Nov 4;291(45):23704-23708. doi: 10.1074/jbc.M116.733857. Epub 2016 Sep 13. J Biol Chem. 2016. PMID: 27624935 Free PMC article.
-
Examining differences in local collagen fiber crimp frequency throughout mechanical testing in a developmental mouse supraspinatus tendon model.J Biomech Eng. 2012 Apr;134(4):041004. doi: 10.1115/1.4006538. J Biomech Eng. 2012. PMID: 22667679 Free PMC article.
References
-
- Atkinson TS, Ewers BJ, Haut RC. The tensile and stress relaxation responses of human patellar tendon varies with specimen cross-sectional area. J Biomech. 1999;32:907–914. - PubMed
-
- Brodsky B, Persikov AV. Molecular structure of the collagen triple helix. Adv Protein Chem. 2005;70:301–359. - PubMed
-
- de Campos Vidal B. Crimp as a part of a helical structure. C R Acad Sci III. 1995;318:173–178. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

