Isolation of a new class of cysteine-glycine-proline-rich beta-proteins (beta-keratins) and their expression in snake epidermis
- PMID: 20070430
- PMCID: PMC2829394
- DOI: 10.1111/j.1469-7580.2009.01192.x
Isolation of a new class of cysteine-glycine-proline-rich beta-proteins (beta-keratins) and their expression in snake epidermis
Abstract
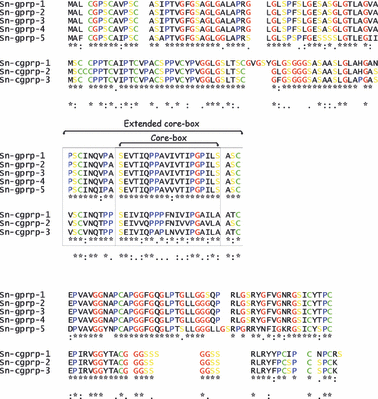
Scales of snakes contain hard proteins (beta-keratins), now referred to as keratin-associated beta-proteins. In the present study we report the isolation, sequencing, and expression of a new group of these proteins from snake epidermis, designated cysteine-glycine-proline-rich proteins. One deduced protein from expressed mRNAs contains 128 amino acids (12.5 kDa) with a theoretical pI at 7.95, containing 10.2% cysteine and 15.6% glycine. The sequences of two more snake cysteine-proline-rich proteins have been identified from genomic DNA. In situ hybridization shows that the messengers for these proteins are present in the suprabasal and early differentiating beta-cells of the renewing scale epidermis. The present study shows that snake scales, as previously seen in scales of lizards, contain cysteine-rich beta-proteins in addition to glycine-rich beta-proteins. These keratin-associated beta-proteins mix with intermediate filament keratins (alpha-keratins) to produce the resistant corneous layer of snake scales. The specific proportion of these two subfamilies of proteins in different scales can determine various degrees of hardness in scales.
Figures






References
-
- Alibardi L. Presence of acid phosphatase in the epidermis of the regenerating tail of the lizard (Podarcis muralis) and its possible role in the process of shedding and keratinization. J Zool. 1998;246:379–390.
-
- Alibardi L. Keratohyalin-like granules in lizard epidermis: evidence from cytochemical, autoradiographic and microanalytic studies. J Morphol. 2001;248:64–79. - PubMed
-
- Alibardi L. Immunocytochemical analysis of the process of keratinization of the epidermis of snakes. J Zool. 2002;258:541–552.
-
- Alibardi L, Sawyer RH. Immunocytochemical analysis of beta keratins in the epidermis of anapsids, lepidosaurians and archosaurians. J Exp Zool. 2002;293:27–38. - PubMed
-
- Alibardi L, Toni M. Cytochemical, biochemical and molecular aspects of the process of keratinization in the epidermis of reptilian scales. Prog. Histochem Cytochem. 2006;40:73–134. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

