Differentiation of human adipose-derived stem cells into beating cardiomyocytes
- PMID: 20070436
- PMCID: PMC3823119
- DOI: 10.1111/j.1582-4934.2010.01009.x
Differentiation of human adipose-derived stem cells into beating cardiomyocytes
Abstract
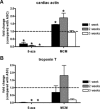
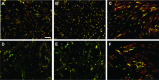
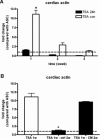
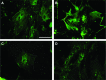
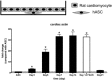
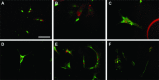
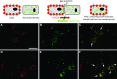
Human adipose-derived stem cells (ASCs) may differentiate into cardiomyocytes and this provides a source of donor cells for tissue engineering. In this study, we evaluated cardiomyogenic differentiation protocols using a DNA demethylating agent 5-azacytidine (5-aza), a modified cardiomyogenic medium (MCM), a histone deacetylase inhibitor trichostatin A (TSA) and co-culture with neonatal rat cardiomyocytes. 5-aza treatment reduced both cardiac actin and TropT mRNA expression. Incubation in MCM only slightly increased gene expression (1.5- to 1.9-fold) and the number of cells co-expressing nkx2.5/sarcomeric alpha-actin (27.2% versus 0.2% in control). TSA treatment increased cardiac actin mRNA expression 11-fold after 1 week, which could be sustained for 2 weeks by culturing cells in cardiomyocyte culture medium. TSA-treated cells also stained positively for cardiac myosin heavy chain, alpha-actin, TropI and connexin43; however, none of these treatments produced beating cells. ASCs in non-contact co-culture showed no cardiac differentiation; however, ASCs co-cultured in direct contact co-culture exhibited a time-dependent increase in cardiac actin mRNA expression (up to 33-fold) between days 3 and 14. Immunocytochemistry revealed co-expression of GATA4 and Nkx2.5, alpha-actin, TropI and cardiac myosin heavy chain in CM-DiI labelled ASCs. Most importantly, many of these cells showed spontaneous contractions accompanied by calcium transients in culture. Human ASC (hASC) showed synchronous Ca(2+) transient and contraction synchronous with surrounding rat cardiomyocytes (106 beats/min.). Gap junctions also formed between them as observed by dye transfer. In conclusion, cell-to-cell interaction was identified as a key inducer for cardiomyogenic differentiation of hASCs. This method was optimized by co-culture with contracting cardiomyocytes and provides a potential cardiac differentiation system to progress applications for cardiac cell therapy or tissue engineering.
Figures
References
-
- World Health Organization . Available at. http://www.who.int/mediacentre/factsheets/fs317/en/index.html. Accessed November 1, 2008.
-
- Eschenhagen T, Zimmermann WH. Engineering myocardial tissue. Circ Res. 2005;97:1220–31. - PubMed
-
- Ott HC, Matthiesen TS, Goh SK, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–21. - PubMed
-
- Zammaretti P, Jaconi M. Cardiac tissue engineering: regeneration of the wounded heart. Curr Opin Biotechnol. 2004;15:430–4. - PubMed
-
- Leor J, Amsalem Y, Cohen S. Cells, scaffolds, and molecules for myocardial tissue engineering. Pharmacol Ther. 2005;105:151–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

