Mechanisms of function of tapasin, a critical major histocompatibility complex class I assembly factor
- PMID: 20070606
- PMCID: PMC2983092
- DOI: 10.1111/j.1600-0854.2009.01025.x
Mechanisms of function of tapasin, a critical major histocompatibility complex class I assembly factor
Abstract
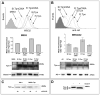
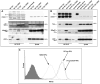
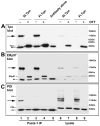
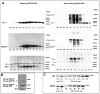
For their efficient assembly in the endoplasmic reticulum (ER), major histocompatibility complex (MHC) class I molecules require the specific assembly factors transporter associated with antigen processing (TAP) and tapasin, as well as generic ER folding factors, including the oxidoreductases ERp57 and protein disulfide isomerase (PDI), and the chaperone calreticulin. TAP transports peptides from the cytosol into the ER. Tapasin promotes the assembly of MHC class I molecules with peptides. The formation of disulfide-linked conjugates of tapasin with ERp57 is suggested to be crucial for tapasin function. Important functional roles are also suggested for the tapasin transmembrane and cytoplasmic domains, sites of tapasin interaction with TAP. We show that interactions of tapasin with both TAP and ERp57 are correlated with strong MHC class I recruitment and assembly enhancement. The presence of the transmembrane/cytosolic regions of tapasin is critical for efficient tapasin-MHC class I binding in interferon-gamma-treated cells, and contributes to an ERp57-independent mode of MHC class I assembly enhancement. A second ERp57-dependent mode of tapasin function correlates with enhanced MHC class I binding to tapasin and calreticulin. We also show that PDI binds to TAP in a tapasin-independent manner, but forms disulfide-linked conjugates with soluble tapasin. Thus, full-length tapasin is important for enhancing recruitment of MHC class I molecules and increasing specificity of tapasin-ERp57 conjugation. Furthermore, tapasin or the TAP/tapasin complex has an intrinsic ability to recruit MHC class I molecules and promote assembly, but also uses generic folding factors to enhance MHC class I recruitment and assembly.
Figures





Similar articles
-
The thiol oxidoreductase ERp57 is a component of the MHC class I peptide-loading complex.Curr Biol. 1998 Jun 4;8(12):709-12. doi: 10.1016/s0960-9822(98)70278-7. Curr Biol. 1998. PMID: 9637923
-
A role for calnexin in the assembly of the MHC class I loading complex in the endoplasmic reticulum.J Immunol. 2001 Feb 1;166(3):1703-9. doi: 10.4049/jimmunol.166.3.1703. J Immunol. 2001. PMID: 11160214
-
Structure of the human MHC-I peptide-loading complex.Nature. 2017 Nov 23;551(7681):525-528. doi: 10.1038/nature24627. Epub 2017 Nov 6. Nature. 2017. PMID: 29107940
-
The role of tapasin in MHC class I antigen assembly.Immunol Res. 1999;20(2):79-88. doi: 10.1007/BF02786464. Immunol Res. 1999. PMID: 10580633 Review.
-
MHC class I assembly: out and about.Trends Immunol. 2008 Sep;29(9):436-43. doi: 10.1016/j.it.2008.06.004. Trends Immunol. 2008. PMID: 18675588 Free PMC article. Review.
Cited by
-
ERAP1 enzyme-mediated trimming and structural analyses of MHC I-bound precursor peptides yield novel insights into antigen processing and presentation.J Biol Chem. 2019 Dec 6;294(49):18534-18544. doi: 10.1074/jbc.RA119.010102. Epub 2019 Oct 10. J Biol Chem. 2019. PMID: 31601650 Free PMC article.
-
Mathematical modeling and stochastic simulations suggest that low-affinity peptides can bisect MHC1-mediated export of high-affinity peptides into "early"- and "late"-phases.Heliyon. 2021 Jul 5;7(7):e07466. doi: 10.1016/j.heliyon.2021.e07466. eCollection 2021 Jul. Heliyon. 2021. PMID: 34286133 Free PMC article.
-
A secreted Tapasin isoform impairs cytotoxic T lymphocyte recognition by disrupting exogenous MHC class I antigen presentation.Front Immunol. 2025 Mar 18;15:1525136. doi: 10.3389/fimmu.2024.1525136. eCollection 2024. Front Immunol. 2025. PMID: 40171019 Free PMC article.
-
Fc Gamma Receptors and Their Role in Antigen Uptake, Presentation, and T Cell Activation.Front Immunol. 2020 Jul 3;11:1393. doi: 10.3389/fimmu.2020.01393. eCollection 2020. Front Immunol. 2020. PMID: 32719679 Free PMC article. Review.
-
Distinct assembly profiles of HLA-B molecules.J Immunol. 2014 Jun 1;192(11):4967-76. doi: 10.4049/jimmunol.1301670. Epub 2014 Apr 30. J Immunol. 2014. PMID: 24790147 Free PMC article.
References
-
- Ortmann B, Copeman J, Lehner PJ, Sadasivan B, Herberg JA, Grandea AG, Riddell SR, Tampe R, Spies T, Trowsdale J, Cresswell P. A critical role for tapasin in the assembly and function of multimeric MHC class I-TAP complexes. Science. 1997;277(5330):1306–1309. - PubMed
-
- Lehner PJ, Surman MJ, Cresswell P. Soluble tapasin restores MHC class I expression and function in the tapasin-negative cell line. 220. Immunity. 1998;8(2):221–231. - PubMed
-
- Garbi N, Tiwari N, Momburg F, Hammerling GJ. A major role for tapasin as a stabilizer of the TAP peptide transporter and consequences for MHC class I expression. Eur J Immunol. 2003;33(1):264–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

