Blunting effect of hypoxia on the proliferation and differentiation of human primary and rat L6 myoblasts is not counteracted by Epo
- PMID: 20070732
- PMCID: PMC6496152
- DOI: 10.1111/j.1365-2184.2009.00648.x
Blunting effect of hypoxia on the proliferation and differentiation of human primary and rat L6 myoblasts is not counteracted by Epo
Abstract
Objectives: The aim of this study was to evaluate whether hypoxia and/or erythropoietin would be able to modulate proliferation/differentiation processes of rat and human myoblasts.
Materials and methods: Rat L6 and primary human myoblasts were grown in 21% or 1% O(2) in the presence or absence of recombinant human erythropoietin (RhEpo). Presence of erythropoietin receptors (EpoR) was assayed using RT-PCR and Western blotting techniques. Cell proliferation was evaluated by determining the doubling time and kinetics of cultures by counting cells. Cell differentiation was analysed by determining myogenic fusion index using antibodies against the myosin heavy chain. Expression of myogenin and myosin heavy chain (MHC) proteins were evaluated using the Western blotting technique.
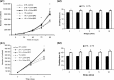
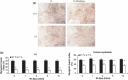
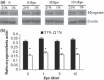
Results: After 96 h culture in growth medium for 2.5 and 9 h, doubling time of L6 and human primary myoblasts respectively, had increased in 1% O(2) conditions (P < 0.01). Kinetics of culture showed alteration in proliferation at 72 h in L6 myoblast cultures and at 4 days in human primary myoblasts. The myogenic fusion index had reduced by 30% in L6 myoblasts and by 20% in human myoblasts (P < 0.01). Expression of myogenin and MHC had reduced by around 50%. Despite presence of EpoR mRNA and protein, RhEpo did not counteract the effects of hypoxia either in L6 cells or in human myoblasts.
Conclusions: The data show that exposure to hypoxic conditions (1% O(2)) of rat and human myoblasts altered their proliferation and differentiation processes. They also show that Epo is not an efficient growth factor to counteract this deleterious effect.
Figures
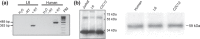

Similar articles
-
Oxygen modulates the glutathione peroxidase activity during the L6 myoblast early differentiation process.Cell Physiol Biochem. 2014;33(1):67-77. doi: 10.1159/000356650. Epub 2014 Jan 3. Cell Physiol Biochem. 2014. PMID: 24401635
-
Erythropoietin stimulates proliferation and interferes with differentiation of myoblasts.J Biol Chem. 2000 Dec 15;275(50):39754-61. doi: 10.1074/jbc.M004999200. J Biol Chem. 2000. PMID: 10995753
-
Hypoxia affects positively the proliferation of bovine satellite cells and their myogenic differentiation through up-regulation of MyoD.Cell Biol Int. 2008 Aug;32(8):871-8. doi: 10.1016/j.cellbi.2008.03.017. Epub 2008 Apr 8. Cell Biol Int. 2008. PMID: 18468460
-
Erythropoietin (EPO)-receptor signaling induces cell death of primary myeloma cells in vitro.J Hematol Oncol. 2016 Aug 31;9(1):75. doi: 10.1186/s13045-016-0306-x. J Hematol Oncol. 2016. PMID: 27581518 Free PMC article. Clinical Trial.
-
EPO-independent functional EPO receptor in breast cancer enhances estrogen receptor activity and promotes cell proliferation.Biochem Biophys Res Commun. 2014 Feb 28;445(1):163-9. doi: 10.1016/j.bbrc.2014.01.165. Epub 2014 Feb 3. Biochem Biophys Res Commun. 2014. PMID: 24502950
Cited by
-
Anemic hypoxemia reduces myoblast proliferation and muscle growth in late-gestation fetal sheep.Am J Physiol Regul Integr Comp Physiol. 2021 Sep 1;321(3):R352-R363. doi: 10.1152/ajpregu.00342.2020. Epub 2021 Jul 21. Am J Physiol Regul Integr Comp Physiol. 2021. PMID: 34287074 Free PMC article.
-
The miR-6240 target gene Igf2bp3 promotes myoblast fusion by enhancing myomaker mRNA stability.Cell Mol Biol Lett. 2024 Dec 5;29(1):152. doi: 10.1186/s11658-024-00650-1. Cell Mol Biol Lett. 2024. PMID: 39639214 Free PMC article.
-
Low Oxygen Tension Enhances Expression of Myogenic Genes When Human Myoblasts Are Activated from G0 Arrest.PLoS One. 2016 Jul 21;11(7):e0158860. doi: 10.1371/journal.pone.0158860. eCollection 2016. PLoS One. 2016. PMID: 27442119 Free PMC article.
-
Insights from the use of erythropoietin in experimental Chagas disease.Int J Parasitol Drugs Drug Resist. 2022 Aug;19:65-80. doi: 10.1016/j.ijpddr.2022.05.005. Epub 2022 Jun 17. Int J Parasitol Drugs Drug Resist. 2022. PMID: 35772309 Free PMC article.
-
Evaluation of functional erythropoietin receptor status in skeletal muscle in vivo: acute and prolonged studies in healthy human subjects.PLoS One. 2012;7(2):e31857. doi: 10.1371/journal.pone.0031857. Epub 2012 Feb 22. PLoS One. 2012. PMID: 22384088 Free PMC article.
References
-
- Scholz D, Thomas S, Sass S, Podzuweit T (2003) Angiogenesis and myogenesis as two facets of inflammatory post‐ischemic tissue regeneration. Mol. Cell. Biochem. 246, 57–67. - PubMed
-
- Di Carlo A, De Mori R, Martelli F, Pompilio G, Capogrossi MC, Germani A (2004) Hypoxia inhibits myogenic differentiation through accelerated MyoD degradation. J. Biol. Chem. 279, 16332–16338. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

