Switching from oral cholinesterase inhibitors to the rivastigmine transdermal patch
- PMID: 20070789
- PMCID: PMC6493826
- DOI: 10.1111/j.1755-5949.2009.00119.x
Switching from oral cholinesterase inhibitors to the rivastigmine transdermal patch
Abstract
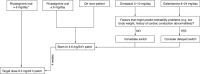
Oral cholinesterase inhibitors (ChEIs) are associated with side effects such as nausea and vomiting. The use of transdermal patches for ChEI delivery may help to minimize these problems. The objective of this review was to consider available data from patients switching from oral ChEIs to transdermal rivastigmine treatment, and to suggest practical guidelines for patients wishing to do this. Literature database and reference list searches were performed to identify suitable publications. Data from two clinical trials and a series of open observational studies, in which patients were switched to the rivastigmine patch from oral rivastigmine, donepezil tablets, or galantamine, were evaluated. Adverse events were tabulated. In the studies reported here, nausea was reported in up to 3.2% and vomiting in up to 1.9% of patients switching to the rivastigmine patch from oral rivastigmine. Similar rates (up to 3.8% of patients for nausea and 0.8% of patients for vomiting) were reported when switching to the rivastigmine patch from donepezil tablets, and no nausea or vomiting was reported in a case study of patients switching to the rivastigmine patch from galantamine tablets. Switching regimes used in clinical trials appeared well tolerated. Data support recommendations for patients on high rivastigmine capsule doses to switch directly to the 9.5 mg/24 h rivastigmine patch, while those on lower oral rivastigmine doses should start on the 4.6 mg/24 h patch for 4 weeks before increasing to the 9.5 mg/24 h patch. This latter regimen is recommended for patients on other oral cholinesterase inhibitors if switching is medically indicated or requested by the patient or the caregiver.
Figures



Similar articles
-
Safety and tolerability of rivastigmine transdermal patch compared with rivastigmine capsules in patients switched from donepezil: data from three clinical trials.Int J Clin Pract. 2010 Jan;64(2):188-93. doi: 10.1111/j.1742-1241.2009.02253.x. Int J Clin Pract. 2010. PMID: 20089009
-
Safety and tolerability of donepezil, rivastigmine and galantamine for patients with Alzheimer's disease: systematic review of the 'real-world' evidence.Dement Geriatr Cogn Disord. 2009;28(5):389-403. doi: 10.1159/000255578. Epub 2009 Nov 6. Dement Geriatr Cogn Disord. 2009. PMID: 19893314
-
Patient adherence to transdermal rivastigmine after switching from oral donepezil: a retrospective claims database study.Alzheimer Dis Assoc Disord. 2013 Apr-Jun;27(2):182-6. doi: 10.1097/WAD.0b013e318266fb02. Alzheimer Dis Assoc Disord. 2013. PMID: 22892648
-
Comparison of cholinesterase inhibitor utilization patterns and associated health care costs in Alzheimer's disease.J Manag Care Pharm. 2008 Jun;14(5):451-61. doi: 10.18553/jmcp.2008.14.5.451. J Manag Care Pharm. 2008. PMID: 18597574 Free PMC article.
-
Use of rivastigmine transdermal patch in the treatment of Alzheimer's disease.Expert Opin Drug Deliv. 2008 Dec;5(12):1377-86. doi: 10.1517/17425240802542690. Expert Opin Drug Deliv. 2008. PMID: 19040398 Review.
Cited by
-
Rivastigmine Patch in Chinese Patients with Probable Alzheimer's disease: A 24-week, Randomized, Double-Blind Parallel-Group Study Comparing Rivastigmine Patch (9.5 mg/24 h) with Capsule (6 mg Twice Daily).CNS Neurosci Ther. 2016 Jun;22(6):488-96. doi: 10.1111/cns.12521. Epub 2016 Mar 25. CNS Neurosci Ther. 2016. PMID: 27012596 Free PMC article. Clinical Trial.
-
Dementia: What pharmacists need to know.Can Pharm J (Ott). 2017 Feb 7;150(2):118-129. doi: 10.1177/1715163517690745. eCollection 2017 Mar-Apr. Can Pharm J (Ott). 2017. PMID: 28405256 Free PMC article. No abstract available.
-
Overcoming barriers to patient adherence: the case for developing innovative drug delivery systems.Nat Rev Drug Discov. 2023 May;22(5):387-409. doi: 10.1038/s41573-023-00670-0. Epub 2023 Mar 27. Nat Rev Drug Discov. 2023. PMID: 36973491 Free PMC article. Review.
-
Strategies for Continued Successful Treatment in Patients with Alzheimer's Disease: An Overview of Switching Between Pharmacological Agents.Curr Alzheimer Res. 2018;15(10):964-974. doi: 10.2174/1567205015666180613112040. Curr Alzheimer Res. 2018. PMID: 29895249 Free PMC article. Review.
-
Hallucinations in Older Adults: A Practical Review.Schizophr Bull. 2020 Dec 1;46(6):1382-1395. doi: 10.1093/schbul/sbaa073. Schizophr Bull. 2020. PMID: 32638012 Free PMC article. Review.
References
-
- Rosenstein LD. Differential diagnosis of the major progressive dementias and depression in middle and late adulthood: A summary of the literature of the early 1990s. Neuropsychol Rev 1998;8:109–167. - PubMed
-
- Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer's disease. Lancet 1976;2:1403. - PubMed
-
- Cummings JL, Kaufer D. Neuropsychiatric aspects of Alzheimer's disease: The cholinergic hypothesis revisited. Neurology 1996;47:876–883. - PubMed
-
- Doody RS, Stevens JC, Beck C, et al Practice parameter: Management of dementia (an evidence‐based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001;56:1154–1166. - PubMed
-
- Enz A, Boddeke H, Gray J, Spiegel R. Pharmacologic and clinicopharmacologic properties of SDZ ENA 713, a centrally selective acetylcholinesterase inhibitor. Ann N Y Acad Sci 1991;640:272–275. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

