Chloroplast-derived enzyme cocktails hydrolyse lignocellulosic biomass and release fermentable sugars
- PMID: 20070870
- PMCID: PMC2854225
- DOI: 10.1111/j.1467-7652.2009.00486.x
Chloroplast-derived enzyme cocktails hydrolyse lignocellulosic biomass and release fermentable sugars
Abstract
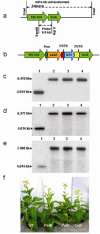
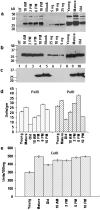
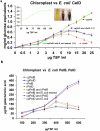
It is widely recognized that biofuel production from lignocellulosic materials is limited by inadequate technology to efficiently and economically release fermentable sugars from the complex multi-polymeric raw materials. Therefore, endoglucanases, exoglucanase, pectate lyases, cutinase, swollenin, xylanase, acetyl xylan esterase, beta glucosidase and lipase genes from bacteria or fungi were expressed in Escherichia coli or tobacco chloroplasts. A PCR-based method was used to clone genes without introns from Trichoderma reesei genomic DNA. Homoplasmic transplastomic lines showed normal phenotype and were fertile. Based on observed expression levels, up to 49, 64 and 10, 751 million units of pectate lyases or endoglucanase can be produced annually, per acre of tobacco. Plant production cost of endoglucanase is 3100-fold, and pectate lyase is 1057 or 1480-fold lower than the same recombinant enzymes sold commercially, produced via fermentation. Chloroplast-derived enzymes had higher temperature stability and wider pH optima than enzymes expressed in E. coli. Plant crude-extracts showed higher enzyme activity than E. coli with increasing protein concentration, demonstrating their direct utility without purification. Addition of E. coli extracts to the chloroplast-derived enzymes significantly decreased their activity. Chloroplast-derived crude-extract enzyme cocktails yielded more (up to 3625%) glucose from filter paper, pine wood or citrus peel than commercial cocktails. Furthermore, pectate lyase transplastomic plants showed enhanced resistance to Erwina soft rot. This is the first report of using plant-derived enzyme cocktails for production of fermentable sugars from lignocellulosic biomass. Limitations of higher cost and lower production capacity of fermentation systems are addressed by chloroplast-derived enzyme cocktails.
Figures



Similar articles
-
Expression of fungal cutinase and swollenin in tobacco chloroplasts reveals novel enzyme functions and/or substrates.PLoS One. 2013;8(2):e57187. doi: 10.1371/journal.pone.0057187. Epub 2013 Feb 25. PLoS One. 2013. PMID: 23451186 Free PMC article.
-
Expression of Trichoderma reesei β-mannanase in tobacco chloroplasts and its utilization in lignocellulosic woody biomass hydrolysis.PLoS One. 2011;6(12):e29302. doi: 10.1371/journal.pone.0029302. Epub 2011 Dec 28. PLoS One. 2011. PMID: 22216240 Free PMC article.
-
Characterization of glycoside hydrolase family 11 xylanase from Streptomyces sp. strain J103; its synergetic effect with acetyl xylan esterase and enhancement of enzymatic hydrolysis of lignocellulosic biomass.Microb Cell Fact. 2021 Jul 8;20(1):129. doi: 10.1186/s12934-021-01619-x. Microb Cell Fact. 2021. PMID: 34238305 Free PMC article.
-
Bioconversion of lignocellulosic biomass: biochemical and molecular perspectives.J Ind Microbiol Biotechnol. 2008 May;35(5):377-391. doi: 10.1007/s10295-008-0327-8. Epub 2008 Mar 13. J Ind Microbiol Biotechnol. 2008. PMID: 18338189 Review.
-
Time dependence of enzyme synergism during the degradation of model and natural lignocellulosic substrates.Enzyme Microb Technol. 2017 Aug;103:1-11. doi: 10.1016/j.enzmictec.2017.04.007. Epub 2017 Apr 25. Enzyme Microb Technol. 2017. PMID: 28554379 Review.
Cited by
-
Expression of fungal cutinase and swollenin in tobacco chloroplasts reveals novel enzyme functions and/or substrates.PLoS One. 2013;8(2):e57187. doi: 10.1371/journal.pone.0057187. Epub 2013 Feb 25. PLoS One. 2013. PMID: 23451186 Free PMC article.
-
Heterologous expression of plant cell wall degrading enzymes for effective production of cellulosic biofuels.J Biomed Biotechnol. 2012;2012:405842. doi: 10.1155/2012/405842. Epub 2012 Jul 15. J Biomed Biotechnol. 2012. PMID: 22911272 Free PMC article. Review.
-
Overexpression of an acidic endo-β-1,3-1,4-glucanase in transgenic maize seed for direct utilization in animal feed.PLoS One. 2013 Dec 31;8(12):e81993. doi: 10.1371/journal.pone.0081993. eCollection 2013. PLoS One. 2013. PMID: 24391711 Free PMC article.
-
High-level expression of a suite of thermostable cell wall-degrading enzymes from the chloroplast genome.Plant Mol Biol. 2011 Jul;76(3-5):311-21. doi: 10.1007/s11103-011-9742-8. Epub 2011 Feb 6. Plant Mol Biol. 2011. PMID: 21298465
-
Plastid Transformation: New Challenges in the Circular Economy Era.Int J Mol Sci. 2022 Dec 3;23(23):15254. doi: 10.3390/ijms232315254. Int J Mol Sci. 2022. PMID: 36499577 Free PMC article. Review.
References
-
- Bae H-J, Kim HJ, Kim YS. Production of recombinant xylanase in plants and its potential for pulp biobleaching applications. Bioresour. Technol. 2008;99:3513–3519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

