Protection against Pseudomonas aeruginosa lung infection in mice by recombinant OprF-pulsed dendritic cell immunization
- PMID: 20070893
- PMCID: PMC2820439
- DOI: 10.1186/1471-2180-10-9
Protection against Pseudomonas aeruginosa lung infection in mice by recombinant OprF-pulsed dendritic cell immunization
Abstract
Background: The Pseudomonas aeruginosa major constitutive outer membrane porin protein F (OprF) has been shown to be a protective antigen and was previously used to activate an immunological response in a mouse model of lung pneumonia. The purpose of our study was to demonstrate the ability of mouse dendritic cells pulsed with purified or recombinant OprF to protect mice against P. aeruginosa infection and inflammation.Both native (n-OprF), isolated and purified from PAO1 bacterial strain, and recombinant (histidin-conjugated) OprF (His-OprF), obtained by cloning of the oprF gene into the pET28a expression vector, were used to stimulate dendritic cells in vitro before adoptive transfer into prospective recipient mice with P. aeruginosa pulmonary infection.
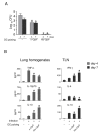
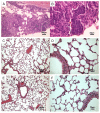
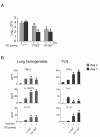
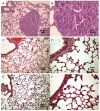
Results: Similar to n-OprF, His-OprF activated dendritic cells in vitro, inducing the costimulatory molecule expression as well as cytokine production. Upon adoptive transfer in vivo, porin-pulsed dendritic cells (DCs) induced Th1-mediated resistance to infection and associated inflammatory pathology caused by either the PAO1 strain or a clinically-isolated mucoid strain.
Conclusions: This study highlights the pivotal contribution of DCs to vaccine-induced protection against P. aeruginosa infection and associated inflammation.
Figures


Similar articles
-
Immunization with outer membrane proteins (OprF and OprI) and flagellin B protects mice from pulmonary infection with mucoid and nonmucoid Pseudomonas aeruginosa.J Microbiol Immunol Infect. 2018 Jun;51(3):312-320. doi: 10.1016/j.jmii.2016.08.014. Epub 2017 Feb 20. J Microbiol Immunol Infect. 2018. PMID: 28291719
-
Protective anti-Pseudomonas aeruginosa humoral and cellular mucosal immunity by AdC7-mediated expression of the P. aeruginosa protein OprF.Vaccine. 2011 Mar 3;29(11):2131-9. doi: 10.1016/j.vaccine.2010.12.087. Epub 2011 Jan 6. Vaccine. 2011. PMID: 21215829 Free PMC article.
-
Protection against Pseudomonas aeruginosa chronic lung infection in mice by genetic immunization against outer membrane protein F (OprF) of P. aeruginosa.Infect Immun. 2001 May;69(5):3510-5. doi: 10.1128/IAI.69.5.3510-3515.2001. Infect Immun. 2001. PMID: 11292786 Free PMC article.
-
Potential of protein OprF of Pseudomonas in bivalent vaccines.Behring Inst Mitt. 1997 Feb;(98):283-90. Behring Inst Mitt. 1997. PMID: 9382752 Review.
-
Vaccines for Pseudomonas aeruginosa: a long and winding road.Expert Rev Vaccines. 2014 Apr;13(4):507-19. doi: 10.1586/14760584.2014.890053. Epub 2014 Feb 27. Expert Rev Vaccines. 2014. PMID: 24575895 Free PMC article. Review.
Cited by
-
Construction, expression, purification and characterization of secretin domain of PilQ and triple PilA-related disulfide loop peptides fusion protein from Pseudomonas aeruginosa.Iran J Basic Med Sci. 2017 May;20(5):458-466. doi: 10.22038/IJBMS.2017.8667. Iran J Basic Med Sci. 2017. PMID: 28656079 Free PMC article.
-
PA0833 Is an OmpA C-Like Protein That Confers Protection Against Pseudomonas aeruginosa Infection.Front Microbiol. 2018 May 23;9:1062. doi: 10.3389/fmicb.2018.01062. eCollection 2018. Front Microbiol. 2018. PMID: 29875759 Free PMC article.
-
Molecular Characterization and Designing of a Novel Multiepitope Vaccine Construct Against Pseudomonas aeruginosa.Int J Pept Res Ther. 2022;28(2):49. doi: 10.1007/s10989-021-10356-z. Epub 2022 Jan 17. Int J Pept Res Ther. 2022. PMID: 35069055 Free PMC article.
-
Pseudomonas aeruginosa Vaccine Development: Lessons, Challenges, and Future Innovations.Int J Mol Sci. 2025 Feb 25;26(5):2012. doi: 10.3390/ijms26052012. Int J Mol Sci. 2025. PMID: 40076637 Free PMC article. Review.
-
Novel cationic peptide TP359 down-regulates the expression of outer membrane biogenesis genes in Pseudomonas aeruginosa: a potential TP359 anti-microbial mechanism.BMC Microbiol. 2016 Aug 22;16(1):192. doi: 10.1186/s12866-016-0808-2. BMC Microbiol. 2016. PMID: 27549081 Free PMC article.
References
-
- Kohlenberg A, Schwab F, Geffers C, Behnke M, Ruden H, Gastmeier P. Time-trends for Gram-negative and multidrug-resistant Gram-positive bacteria associated with nosocomial infections in German intensive care units between 2000 and 2005. Clin Microbiol Infect. 2008;14:93–96. doi: 10.1111/j.1469-0691.2007.01879.x. - DOI - PubMed
-
- Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

