Senescent synapses and hippocampal circuit dynamics
- PMID: 20071039
- PMCID: PMC3076741
- DOI: 10.1016/j.tins.2009.12.003
Senescent synapses and hippocampal circuit dynamics
Abstract
Excitatory synaptic transmission is altered during aging in hippocampal granule cells, and in CA3 and CA1 pyramidal cells. These functional changes contribute to age-associated impairments in experimentally-induced plasticity in each of these primary hippocampal subregions. In CA1, plasticity evoked by stimulation shares common mechanisms with the synaptic modification observed following natural behavior. Aging results in deficits in both artificially- and behaviorally-induced plasticity, and this could in part reflect age-related changes in Ca2+ homeostasis. Other observations, however, suggest that increased intracellular Ca2+ levels are beneficial under some circumstances. This review focuses on age-associated changes in synaptic function, how these alterations might contribute to cognitive decline, and the extent to which altered hippocampal circuit properties are detrimental or reflect compensatory processes.
Copyright 2009 Elsevier Ltd. All rights reserved.
Figures
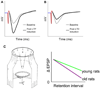
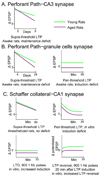


References
-
- Kinsella K, Velkoff VA. An aging world: 2001. U.S. Census Bureau. 2001 Series P95/01-1.
-
- Hebb D. On watching myself get old. Psychology Today. 1978 November
-
- Moscovitch M, et al. The cognitive neuroscience of remote episodic, semantic and spatial memory. Curr Opin Neurobiol. 2006;16(2):179–190. - PubMed
-
- Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog Neurobiol. 2003;69(3):143–179. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

