A rapid and sensitive method for determination of veliparib (ABT-888), in human plasma, bone marrow cells and supernatant by using LC/MS/MS
- PMID: 20071126
- PMCID: PMC2822340
- DOI: 10.1016/j.jpba.2009.12.015
A rapid and sensitive method for determination of veliparib (ABT-888), in human plasma, bone marrow cells and supernatant by using LC/MS/MS
Abstract
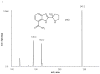
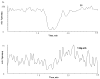
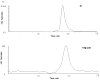
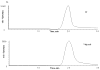
A rapid and sensitive method was developed and validated using a liquid chromatographic method with tandem mass spectrometry detection (LC/MS/MS) for determination of veliparib (ABT-888) in plasma, bone marrow supernatant, and bone marrow cells. Sample preparation involved a single protein precipitation step by the addition of the sample with acetonitrile. Separation of veliparib and the internal standard, A620223.69, was achieved on a Atlantis dC(18) column (100mmx2.1mm, 3microm) column using a mobile phase consisting of acetonitrile-ammonium acetate (2mM) containing formic acid (0.1%, v/v) using isocratic flow at 0.2mL/min for 3min. The analyte and internal standard were monitored by tandem mass spectrometry with electrospray positive ionization. Linear calibration curves were generated over the range of 5-1000nM. The values for both within day and between day precision and accuracy were well within the generally accepted criteria for analytical methods. This method was subsequently used to measure concentrations of veliparib in cancer patients receiving an oral daily dose of 10mg with demonstration of drug accumulation in the marrow compartment and in the target leukemia bone marrow cells.
Copyright 2009 Elsevier B.V. All rights reserved.
Figures






Similar articles
-
A liquid chromatography/tandem mass spectrometry assay to quantitate MS-275 in human plasma.J Pharm Biomed Anal. 2007 Jan 17;43(2):784-7. doi: 10.1016/j.jpba.2006.08.006. Epub 2006 Sep 12. J Pharm Biomed Anal. 2007. PMID: 16971082 Free PMC article.
-
Liquid chromatography-mass spectrometric assay for the quantitation in human plasma of ABT-888, an orally available, small molecule inhibitor of poly(ADP-ribose) polymerase.J Chromatogr B Analyt Technol Biomed Life Sci. 2008 Sep 1;872(1-2):141-7. doi: 10.1016/j.jchromb.2008.07.032. Epub 2008 Jul 31. J Chromatogr B Analyt Technol Biomed Life Sci. 2008. PMID: 18692448 Free PMC article.
-
Simultaneous determination of ABT-888, a poly (ADP-ribose) polymerase inhibitor, and its metabolite in human plasma by liquid chromatography/tandem mass spectrometry.J Chromatogr B Analyt Technol Biomed Life Sci. 2010 Feb 1;878(3-4):333-9. doi: 10.1016/j.jchromb.2009.11.037. Epub 2009 Nov 27. J Chromatogr B Analyt Technol Biomed Life Sci. 2010. PMID: 20005184 Free PMC article. Clinical Trial.
-
A rapid and sensitive method for determination of sorafenib in human plasma using a liquid chromatography/tandem mass spectrometry assay.J Chromatogr B Analyt Technol Biomed Life Sci. 2007 Feb 1;846(1-2):1-7. doi: 10.1016/j.jchromb.2006.06.005. Epub 2006 Jun 23. J Chromatogr B Analyt Technol Biomed Life Sci. 2007. PMID: 16798122
-
Development and validation of a liquid chromatography/tandem mass spectrometry method for the determination of DMXAA in human and mouse plasma.J Chromatogr B Analyt Technol Biomed Life Sci. 2007 Jun 1;852(1-2):217-22. doi: 10.1016/j.jchromb.2007.01.018. Epub 2007 Jan 26. J Chromatogr B Analyt Technol Biomed Life Sci. 2007. PMID: 17307035
Cited by
-
Population pharmacokinetics and site of action exposures of veliparib with topotecan plus carboplatin in patients with haematological malignancies.Br J Clin Pharmacol. 2017 Aug;83(8):1688-1700. doi: 10.1111/bcp.13253. Epub 2017 Mar 19. Br J Clin Pharmacol. 2017. PMID: 28156017 Free PMC article. Clinical Trial.
-
Bone-Seeking Matrix Metalloproteinase-2 Inhibitors Prevent Bone Metastatic Breast Cancer Growth.Mol Cancer Ther. 2017 Mar;16(3):494-505. doi: 10.1158/1535-7163.MCT-16-0315-T. Epub 2017 Jan 9. Mol Cancer Ther. 2017. PMID: 28069877 Free PMC article.
-
A Phase I Study of Topotecan, Carboplatin and the PARP Inhibitor Veliparib in Acute Leukemias, Aggressive Myeloproliferative Neoplasms, and Chronic Myelomonocytic Leukemia.Clin Cancer Res. 2017 Feb 15;23(4):899-907. doi: 10.1158/1078-0432.CCR-16-1274. Epub 2016 Aug 22. Clin Cancer Res. 2017. PMID: 27551000 Free PMC article. Clinical Trial.
-
A Review on Liquid Chromatography-Tandem Mass Spectrometry Methods for Rapid Quantification of Oncology Drugs.Pharmaceutics. 2018 Nov 8;10(4):221. doi: 10.3390/pharmaceutics10040221. Pharmaceutics. 2018. PMID: 30413076 Free PMC article. Review.
References
-
- Curtin NJ. PARP inhibitors for cancer therapy. Expert Rev Mol Med. 2005;7:1–20. - PubMed
-
- Ame JC, Spenlehauer C, de Murcia G. The PARP superfamily. Bioessays. 2004;26:882–893. - PubMed
-
- Jagtap P, Szabo C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005;4:421–440. - PubMed
-
- Low J. Solicitation for preclinical studies (ABT-888 PARP inhibitor) CTEP rapid communication. 2005.
-
- Donawho CK, Luo Y, Luo Y, Penning TD, Bauch JL, Bouska JJ, Bontcheva-Diaz VD, Cox BF, DeWeese TL, Dillehay LE, Ferguson DC, Ghoreishi-Haack NS, Grimm DR, Guan R, Han EK, Holley-Shanks RR, Hristov B, Idler KB, Jarvis K, Johnson EF, Kleinberg LR, Klinghofer V, Lasko LM, Liu X, Marsh KC, McGonigal TP, Meulbroek JA, Olson AM, Palma JP, Rodriguez LE, Shi Y, Stavropoulos JA, Tsurutani AC, Zhu GD, Rosenberg SH, Giranda VL, Frost DJ. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res. 2007;13:2728–2737. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

