Osteopontin is cleaved at multiple sites close to its integrin-binding motifs in milk and is a novel substrate for plasmin and cathepsin D
- PMID: 20071328
- PMCID: PMC2832943
- DOI: 10.1074/jbc.M109.075010
Osteopontin is cleaved at multiple sites close to its integrin-binding motifs in milk and is a novel substrate for plasmin and cathepsin D
Abstract
Osteopontin (OPN) is a highly modified integrin-binding protein present in most tissues and body fluids where it has been implicated in numerous biological processes. A significant regulation of OPN function is mediated through phosphorylation and proteolytic processing. Proteolytic cleavage by thrombin and matrix metalloproteinases close to the integrin-binding Arg-Gly-Asp sequence modulates the function of OPN and its integrin binding properties. In this study, seven N-terminal OPN fragments originating from proteolytic cleavage have been characterized from human milk. Identification of the cleavage sites revealed that all fragments contained the Arg-Gly-Asp(145) sequence and were generated by cleavage of the Leu(151)-Arg(152), Arg(152)-Ser(153), Ser(153)-Lys(154), Lys(154)-Ser(155), Ser(155)-Lys(156), Lys(156)-Lys(157), or Phe(158)-Arg(159) peptide bonds. Six cleavages cannot be ascribed to thrombin or matrix metalloproteinase activity, whereas the cleavage at Arg(152)-Ser(153) matches thrombin specificity for OPN. The principal protease in milk, plasmin, hydrolyzed the same peptide bond as thrombin, but its main cleavage site was identified to be Lys(154)-Ser(155). Another endogenous milk protease, cathepsin D, cleaved the Leu(151)-Arg(152) bond. OPN fragments corresponding to plasmin activity were also identified in urine showing that plasmin cleavage of OPN is not restricted to milk. Plasmin, but not cathepsin D, cleavage of OPN increased cell adhesion mediated by the alpha(V)beta(3)- or alpha(5)beta(1)-integrins. Similar cellular adhesion was mediated by plasmin and thrombin-cleaved OPN showing that plasmin can be a potent regulator of OPN activity. These data show that OPN is highly susceptible to cleavage near its integrin-binding motifs, and the protein is a novel substrate for plasmin and cathepsin D.
Figures





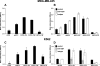
References
-
- Sodek J., Ganss B., McKee M. D. (2000) Crit. Rev. Oral Biol. Med. 11, 279–303 - PubMed
-
- Wang K. X., Denhardt D. T. (2008) Cytokine Growth Factor Rev. 19, 333–345 - PubMed
-
- Schack L., Lange A., Kelsen J., Agnholt J., Christensen B., Petersen T. E., Sørensen E. S. (2009) J. Dairy Sci. 92, 5378–5385 - PubMed
-
- Nemir M., Bhattacharyya D., Li X., Singh K., Mukherjee A. B., Mukherjee B. B. (2000) J. Biol. Chem. 275, 969–976 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

