Force-induced myofibroblast differentiation through collagen receptors is dependent on mammalian diaphanous (mDia)
- PMID: 20071339
- PMCID: PMC2838345
- DOI: 10.1074/jbc.M109.075218
Force-induced myofibroblast differentiation through collagen receptors is dependent on mammalian diaphanous (mDia)
Abstract
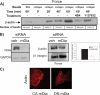
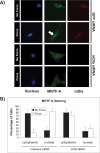
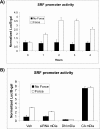
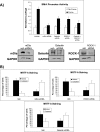
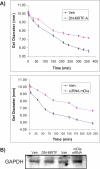
The development of fibrosis promotes the differentiation of myofibroblasts, pro-fibrotic cells, which contribute to tissue dysfunction. Myofibroblast differentiation is dependent on actin assembly, which in response to force, is mediated by various actin-binding proteins including the mammalian Diaphanous-related formins (mDia). We examined the role of mDia in the mechano-sensing pathway that leads to force-induced expression of alpha-smooth muscle actin (SMA), a marker and critical determinant of myofibroblast differentiation. In cells treated with siRNA to knockdown mDia and then subjected to tensile force using collagen-coated magnetite beads attached to beta1 integrins, actin assembly was inhibited at bead contact sites. Force-induced nuclear translocation of MRTF-A, a transcriptional co-activator of SMA, was reduced 50% by mDia knockdown. The expression of the transcriptional co-activator of SMA, serum response factor, was reduced by 50% after siRNA knockdown of mDia or by 100% in cells transfected with catalytically inactive mDia. Force-induced activation of the SMA promoter and SMA expression were blocked by knockdown of siRNA of mDia. In anchored collagen gel assays to measure myofibroblast-mediated contraction, knockdown of mDia reduced contraction by 50%. We conclude that mDia plays an important role in the development of force-induced transcriptional activation of SMA and myofibroblast differentiation.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

