Initiation of purinergic signaling by exocytosis of ATP-containing vesicles in liver epithelium
- PMID: 20071341
- PMCID: PMC2832965
- DOI: 10.1074/jbc.M109.065482
Initiation of purinergic signaling by exocytosis of ATP-containing vesicles in liver epithelium
Abstract
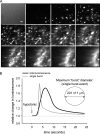
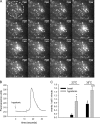
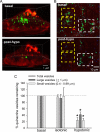
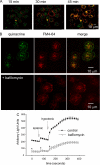
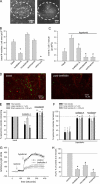
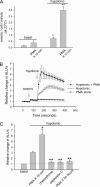
Extracellular ATP represents an important autocrine/paracrine signaling molecule within the liver. The mechanisms responsible for ATP release are unknown, and alternative pathways have been proposed, including either conductive ATP movement through channels or exocytosis of ATP-enriched vesicles, although direct evidence from liver cells has been lacking. Utilizing dynamic imaging modalities (confocal and total internal reflection fluorescence microscopy and luminescence detection utilizing a high sensitivity CCD camera) at different scales, including confluent cell populations, single cells, and the intracellular submembrane space, we have demonstrated in a model liver cell line that (i) ATP release is not uniform but reflects point source release by a defined subset of cells; (ii) ATP within cells is localized to discrete zones of high intensity that are approximately 1 mum in diameter, suggesting a vesicular localization; (iii) these vesicles originate from a bafilomycin A(1)-sensitive pool, are depleted by hypotonic exposure, and are not rapidly replenished from recycling of endocytic vesicles; and (iv) exocytosis of vesicles in response to cell volume changes depends upon a complex series of signaling events that requires intact microtubules as well as phosphoinositide 3-kinase and protein kinase C. Collectively, these findings are most consistent with an essential role for exocytosis in regulated release of ATP and initiation of purinergic signaling in liver cells.
Figures
Similar articles
-
Regulation of purinergic signaling in biliary epithelial cells by exocytosis of SLC17A9-dependent ATP-enriched vesicles.J Biol Chem. 2011 Jul 15;286(28):25363-76. doi: 10.1074/jbc.M111.232868. Epub 2011 May 25. J Biol Chem. 2011. PMID: 21613220 Free PMC article.
-
Vesicular exocytosis contributes to volume-sensitive ATP release in biliary cells.Am J Physiol Gastrointest Liver Physiol. 2004 Apr;286(4):G538-46. doi: 10.1152/ajpgi.00355.2003. Epub 2003 Nov 6. Am J Physiol Gastrointest Liver Physiol. 2004. PMID: 14604861
-
Regulation of cellular ATP release.Trans Am Clin Climatol Assoc. 2007;118:199-208. Trans Am Clin Climatol Assoc. 2007. PMID: 18528503 Free PMC article. Review.
-
Evidence for Gd(3+) inhibition of membrane ATP permeability and purinergic signaling.Am J Physiol. 1999 Dec;277(6):G1222-30. doi: 10.1152/ajpgi.1999.277.6.G1222. Am J Physiol. 1999. PMID: 10600820
-
Basal release of ATP: an autocrine-paracrine mechanism for cell regulation.Sci Signal. 2010 Jan 12;3(104):re1. doi: 10.1126/scisignal.3104re1. Sci Signal. 2010. PMID: 20068232 Free PMC article. Review.
Cited by
-
PKCα regulates TMEM16A-mediated Cl⁻ secretion in human biliary cells.Am J Physiol Gastrointest Liver Physiol. 2016 Jan 1;310(1):G34-42. doi: 10.1152/ajpgi.00146.2015. Epub 2015 Nov 5. Am J Physiol Gastrointest Liver Physiol. 2016. PMID: 26542395 Free PMC article.
-
Intercellular Ca(2+) waves: mechanisms and function.Physiol Rev. 2012 Jul;92(3):1359-92. doi: 10.1152/physrev.00029.2011. Physiol Rev. 2012. PMID: 22811430 Free PMC article. Review.
-
Adenosine triphosphate release and purinergic (P2) receptor-mediated secretion in small and large mouse cholangiocytes.Hepatology. 2010 Nov;52(5):1819-28. doi: 10.1002/hep.23883. Hepatology. 2010. PMID: 20827720 Free PMC article.
-
The role of pannexin hemichannels in inflammation and regeneration.Front Physiol. 2014 Feb 25;5:63. doi: 10.3389/fphys.2014.00063. eCollection 2014. Front Physiol. 2014. PMID: 24616702 Free PMC article. Review.
-
Role of purinergic P2X receptors in the control of liver homeostasis.Wiley Interdiscip Rev Membr Transp Signal. 2012 May;1(3):341-348. doi: 10.1002/wmts.32. Epub 2012 Jan 11. Wiley Interdiscip Rev Membr Transp Signal. 2012. PMID: 22662313 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

