Hepatic heme-regulated inhibitor (HRI) eukaryotic initiation factor 2alpha kinase: a protagonist of heme-mediated translational control of CYP2B enzymes and a modulator of basal endoplasmic reticulum stress tone
- PMID: 20071449
- PMCID: PMC2845940
- DOI: 10.1124/mol.109.061259
Hepatic heme-regulated inhibitor (HRI) eukaryotic initiation factor 2alpha kinase: a protagonist of heme-mediated translational control of CYP2B enzymes and a modulator of basal endoplasmic reticulum stress tone
Retraction in
-
Notice of Retraction: Acharya P, Chen J-J, Correia MA (2010) Hepatic heme-regulated inhibitor (HRI) eukaryotic initiation factor 2α kinase: a protagonist of heme-mediated translational control of CYP2B enzymes and a modulator of basal endoplasmic reticulum stress tone. Mol Pharmacol 77(4):575-592; doi:10.1124/mol.109.061259.Mol Pharmacol. 2021 Aug;100(2):65. doi: 10.1124/mol.109.061259retraction. Mol Pharmacol. 2021. PMID: 34404736 Free PMC article. No abstract available.
Abstract
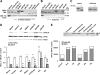
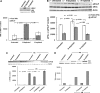
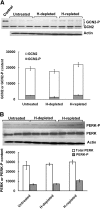
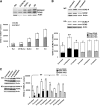
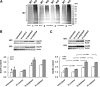
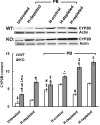
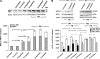
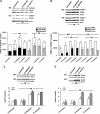
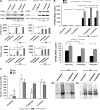
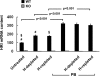
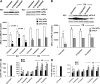
We have reported previously that the hepatic heme-regulated inhibitor (HRI)-eukaryotic initiation factor 2 alpha (eIF2 alpha) kinase is activated in acute heme-deficient states, resulting in translational shut-off of global hepatic protein synthesis, including phenobarbital (PB)-mediated induction of CYP2B enzymes in rats. These findings revealed that heme regulates hepatic CYP2B synthesis at the translational level via HRI. As a proof of concept, we have now employed a genetic HRI-knockout (KO) mouse hepatocyte model. In HRI-KO hepatocytes, PB-mediated CYP2B protein induction is no longer regulated by hepatic heme availability and proceeds undeterred even after acute hepatic heme depletion. It is noteworthy that genetic ablation of HRI led to a small albeit significant elevation of basal hepatic endoplasmic reticulum (ER) stress as revealed by the activation of ER stress-inducible RNA-dependent protein kinase-like ER-integral (PERK) eIF2 alpha-kinase, and induction of hepatic protein ubiquitination and ER chaperones Grp78 and Grp94. Such ER stress was further augmented after PB-mediated hepatic protein induction. These findings suggest that HRI normally modulates the basal hepatic ER stress tone. Furthermore, because HRI exists in both human and rat liver in its heme-sensitive form and is inducible by cytochrome P450 inducers such as PB, these findings are clinically relevant to acute heme-deficient states, such as the acute hepatic porphyrias. Activation of this exquisitely sensitive heme sensor would normally protect cells by safeguarding cellular energy and nutrients during acute heme deficiency. However, similar HRI activation in genetically predisposed persons could lead to global translational arrest of physiologically relevant enzymes and proteins, resulting in the severe and often fatal clinical symptoms of the acute hepatic porphyrias.
Figures
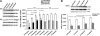
References
-
- Acharya P, Engel JC, Correia MA. (2009) Hepatic CYP3A suppression by high concentrations of proteasomal inhibitors: a consequence of RNA-dependent protein kinase-like ER-bound eukaryotic initiation factor 2alpha (eIF2alpha)-kinase (PERK) and general control nonderepressible-2 eIF2α kinase (GCN2), and global translational shutoff. Mol Pharmacol 76:503–515 - PMC - PubMed
-
- Anderson KE, Bloomer JR, Bonkovsky HL, Kushner JP, Pierach CA, Pimstone NR, Desnick RJ. (2005) Recommendations for the diagnosis and treatment of the acute porphyrias. Ann Intern Med 142:439–450 - PubMed
-
- Berlanga JJ, Herrero S, de Haro C. (1998) Characterization of the hemin-sensitive eukaryotic initiation factor 2alpha kinase from mouse nonerythroid cells. J Biol Chem 273:32340–32346 - PubMed
-
- Chefalo PJ, Oh J, Rafie-Kolpin M, Kan B, Chen JJ. (1998) Heme-regulated eIF-2alpha kinase purifies as a hemoprotein. Eur J Biochem 258:820–830 - PubMed
-
- Chen JJ, Crosby JS, London IM. (1994) Regulation of heme-regulated eIF-2 alpha kinase and its expression in erythroid cells. Biochimie 76:761–769 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

