Dietary fructose causes tubulointerstitial injury in the normal rat kidney
- PMID: 20071464
- PMCID: PMC2838595
- DOI: 10.1152/ajprenal.00433.2009
Dietary fructose causes tubulointerstitial injury in the normal rat kidney
Abstract
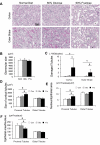
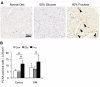
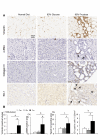
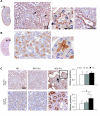
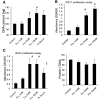
Recent studies suggest that the metabolic syndrome is associated with renal disease. We previously reported that a high-fructose diet, but not a high-glucose diet, can induce metabolic syndrome and accelerate chronic renal disease in rats. We now examined the effects of a high-fructose diet on normal rat kidneys. Three groups of Sprague-Dawley rats were pair fed a special diet containing 60% fructose, 60% glucose, or control standard rat chow for 6 wk, and then histological studies were performed. The effect of fructose to induce cell proliferation in cultured proximal tubular cells was also performed. Fructose diet, but not glucose diet, significantly increased kidney weight by 6 wk. The primary finding was tubular hyperplasia and proliferation involving all segments of the proximal tubules while glomerular changes were not observed. This is the same site where the fructose transporters (GLUT2 and -5) as well as the key enzyme in fructose metabolism (ketohexokinase) were expressed. Consistently, fructose also induced proliferation of rat proximal tubular cells in culture. In vivo, tubular proliferation was also associated with focal tubular injury, with type III collagen deposition in the interstitium, an increase in alpha-smooth muscle actin positive myofibroblasts, and an increase in macrophage infiltration. In conclusion, a high-fructose diet induces cell proliferation and hyperplasia in proximal tubules, perhaps via a direct metabolic effect. The effect is independent of total energy intake and is associated with focal tubulointerstitial injury. These studies may provide a mechanism by which metabolic syndrome causes renal disease.
Figures
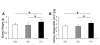

References
-
- Berridge MV, Herst PM, Tan AS. Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol Annu Rev 11: 127–152, 2005 - PubMed
-
- Burant CF, Takeda J, Brot-Laroche E, Bell GI, Davidson NO. Fructose transporter in human spermatozoa and small intestine is GLUT5. J Biol Chem 267: 14523–14526, 1992 - PubMed
-
- Burch HB, Choi S, Dence CN, Alvey TR, Cole BR, Lowry OH. Metabolic effects of large fructose loads in different parts of the rat nephron. J Biol Chem 255: 8239–8244, 1980 - PubMed
-
- Cheeseman CI. GLUT2 is the transporter for fructose across the rat intestinal basolateral membrane. Gastroenterology 105: 1050–1056, 1993 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

