Mutations in Caenorhabditis elegans him-19 show meiotic defects that worsen with age
- PMID: 20071466
- PMCID: PMC2836969
- DOI: 10.1091/mbc.e09-09-0811
Mutations in Caenorhabditis elegans him-19 show meiotic defects that worsen with age
Abstract
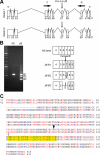
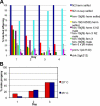
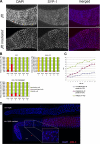
From a screen for meiotic Caenorhabditis elegans mutants based on high incidence of males, we identified a novel gene, him-19, with multiple functions in prophase of meiosis I. Mutant him-19(jf6) animals show a reduction in pairing of homologous chromosomes and subsequent bivalent formation. Consistently, synaptonemal complex formation is spatially restricted and possibly involves nonhomologous chromosomes. Also, foci of the recombination protein RAD-51 occur delayed or cease altogether. Ultimately, mutation of him-19 leads to chromosome missegregation and reduced offspring viability. The observed defects suggest that HIM-19 is important for both homology recognition and formation of meiotic DNA double-strand breaks. It therefore seems to be engaged in an early meiotic event, resembling in this respect the regulator kinase CHK-2. Most astonishingly, him-19(jf6) hermaphrodites display worsening of phenotypes with increasing age, whereas defects are more severe in female than in male meiosis. This finding is consistent with depletion of a him-19-dependent factor during the production of oocytes. Further characterization of him-19 could contribute to our understanding of age-dependent meiotic defects in humans.
Figures




Similar articles
-
A C. elegans eIF4E-family member upregulates translation at elevated temperatures of mRNAs encoding MSH-5 and other meiotic crossover proteins.J Cell Sci. 2010 Jul 1;123(Pt 13):2228-37. doi: 10.1242/jcs.063107. Epub 2010 Jun 8. J Cell Sci. 2010. PMID: 20530576 Free PMC article.
-
Caenorhabditis elegans prom-1 is required for meiotic prophase progression and homologous chromosome pairing.Mol Biol Cell. 2007 Dec;18(12):4911-20. doi: 10.1091/mbc.e07-03-0243. Epub 2007 Oct 3. Mol Biol Cell. 2007. PMID: 17914060 Free PMC article.
-
Meiotic Double-Strand Break Processing and Crossover Patterning Are Regulated in a Sex-Specific Manner by BRCA1-BARD1 in Caenorhabditis elegans.Genetics. 2020 Oct;216(2):359-379. doi: 10.1534/genetics.120.303292. Epub 2020 Aug 12. Genetics. 2020. PMID: 32796008 Free PMC article.
-
Meiotic recombination in Caenorhabditis elegans.Chromosome Res. 2007;15(5):607-21. doi: 10.1007/s10577-007-1146-x. Chromosome Res. 2007. PMID: 17674149 Review.
-
Homologue pairing, recombination and segregation in Caenorhabditis elegans.Genome Dyn. 2009;5:43-55. doi: 10.1159/000166618. Genome Dyn. 2009. PMID: 18948706 Review.
Cited by
-
Whole genome sequencing facilitates intragenic variant interpretation following modifier screening in C. elegans.BMC Genomics. 2021 Nov 13;22(1):820. doi: 10.1186/s12864-021-08142-8. BMC Genomics. 2021. PMID: 34773966 Free PMC article.
-
DNA double-strand break repair in Caenorhabditis elegans.Chromosoma. 2011 Feb;120(1):1-21. doi: 10.1007/s00412-010-0296-3. Epub 2010 Nov 5. Chromosoma. 2011. PMID: 21052706 Free PMC article. Review.
-
A new thermosensitive smc-3 allele reveals involvement of cohesin in homologous recombination in C. elegans.PLoS One. 2011;6(9):e24799. doi: 10.1371/journal.pone.0024799. Epub 2011 Sep 21. PLoS One. 2011. PMID: 21957461 Free PMC article.
-
Initiation of Meiotic Development Is Controlled by Three Post-transcriptional Pathways in Caenorhabditis elegans.Genetics. 2018 Aug;209(4):1197-1224. doi: 10.1534/genetics.118.300985. Epub 2018 Jun 25. Genetics. 2018. PMID: 29941619 Free PMC article.
-
Overlapping and separable activities of BRA-2 and HIM-17 promote occurrence and regulation of pairing and synapsis during Caenorhabditis elegans meiosis.Nat Commun. 2025 Mar 13;16(1):2516. doi: 10.1038/s41467-025-57862-y. Nat Commun. 2025. PMID: 40082424 Free PMC article.
References
-
- Alpi A., Pasierbek P., Gartner A., Loidl J. Genetic and cytological characterization of the recombination protein RAD-51 in Caenorhabditis elegans. Chromosoma. 2003;112:6–16. - PubMed
-
- Colaiacovo M. P., MacQueen A. J., Martinez-Perez E., McDonald K., Adamo A., La Volpe A., Villeneuve A. M. Synaptonemal complex assembly in C. elegans is dispensable for loading strand-exchange proteins but critical for proper completion of recombination. Dev. Cell. 2003;5:463–474. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

