Evolutionary gain of function for the ER membrane protein Sec62 from yeast to humans
- PMID: 20071467
- PMCID: PMC2828957
- DOI: 10.1091/mbc.e09-08-0730
Evolutionary gain of function for the ER membrane protein Sec62 from yeast to humans
Abstract
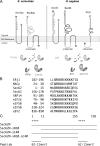
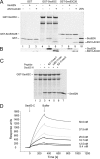
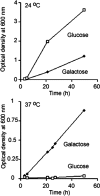
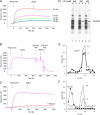
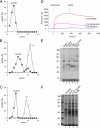
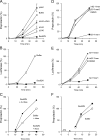
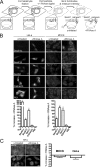
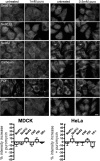
Because of similarity to their yeast orthologues, the two membrane proteins of the human endoplasmic reticulum (ER) Sec62 and Sec63 are expected to play a role in protein biogenesis in the ER. We characterized interactions between these two proteins as well as the putative interaction of Sec62 with ribosomes. These data provide further evidence for evolutionary conservation of Sec62/Sec63 interaction. In addition, they indicate that in the course of evolution Sec62 of vertebrates has gained an additional function, the ability to interact with the ribosomal tunnel exit and, therefore, to support cotranslational mechanisms such as protein transport into the ER. This view is supported by the observation that Sec62 is associated with ribosomes in human cells. Thus, the human Sec62/Sec63 complex and the human ER membrane protein ERj1 are similar in providing binding sites for BiP in the ER-lumen and binding sites for ribosomes in the cytosol. We propose that these two systems provide similar chaperone functions with respect to different precursor proteins.
Figures


References
-
- Beckmann R., Spahn C.M.T., Eswar N., Helmers J., Penczek P., Sali A., Frank J., Blobel G. Architecture of the protein-conducting channel associated with the translating 80S ribosome. Cell. 2001;107:361–372. - PubMed
-
- Blau M., Mullapudi S., Becker T., Dudek J., Zimmermann R., Penczek P., Beckmann R. ERj1p uses a universal ribosomal adaptor site to coordinate the 80S ribosome at the membrane. Nat. Struct. Mol. Biol. 2005;12:1015–1016. - PubMed
-
- Davila S., et al. Mutations in SEC63 cause autosomal dominant polycystic liver disease. Nat. Genet. 2004;36:575–577. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

