Postsynaptic clustering and activation of Pyk2 by PSD-95
- PMID: 20071509
- PMCID: PMC2822408
- DOI: 10.1523/JNEUROSCI.4992-08.2010
Postsynaptic clustering and activation of Pyk2 by PSD-95
Abstract
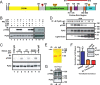
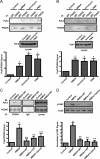
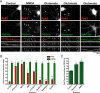
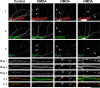
The tyrosine kinase Pyk2 plays a unique role in intracellular signal transduction by linking Ca(2+) influx to tyrosine phosphorylation, but the molecular mechanism of Pyk2 activation is unknown. We report that Pyk2 oligomerization by antibodies in vitro or overexpression of PSD-95 in PC6-3 cells induces trans-autophosphorylation of Tyr402, the first step in Pyk2 activation. In neurons, Ca(2+) influx through NMDA-type glutamate receptors causes postsynaptic clustering and autophosphorylation of endogenous Pyk2 via Ca(2+)- and calmodulin-stimulated binding to PSD-95. Accordingly, Ca(2+) influx promotes oligomerization and thereby autoactivation of Pyk2 by stimulating its interaction with PSD-95. We show that this mechanism of Pyk2 activation is critical for long-term potentiation in the hippocampus CA1 region, which is thought to underlie learning and memory.
Figures


References
-
- Ahmed R, Zha XM, Green SH, Dailey ME. Synaptic activity and F-actin coordinately regulate CaMKIIalpha localization to dendritic postsynaptic sites in developing hippocampal slices. Mol Cell Neurosci. 2006;31:37–51. - PubMed
-
- Akyol Z, Bartos JA, Merrill MA, Faga LA, Jaren OR, Shea MA, Hell JW. Apo-calmodulin binds with its C-terminal domain to the N-methyl-D-aspartate receptor NR1 C0 region. J Biol Chem. 2004;279:2166–2175. - PubMed
-
- Altrock WD, tom Dieck S, Sokolov M, Meyer AC, Sigler A, Brakebusch C, Fässler R, Richter K, Boeckers TM, Potschka H, Brandt C, Löscher W, Grimberg D, Dresbach T, Hempelmann A, Hassan H, Balschun D, Frey JU, Brandstätter JH, Garner CC, Rosenmund C, Gundelfinger ED. Functional inactivation of a fraction of excitatory synapses in mice deficient for the active zone protein bassoon. Neuron. 2003;37:787–800. - PubMed
-
- Avraham H, Park SY, Schinkmann K, Avraham S. RAFTK/Pyk2-mediated cellular signalling. Cell Signal. 2000;12:123–133. - PubMed
-
- Avraham S, London R, Fu Y, Ota S, Hiregowdara D, Li J, Jiang S, Pasztor LM, White RA, Groopman JE, Avraham H. Identification and characterization of a novel related adhesion focal tyrosine kinase (RAFTK) from megakaryocytes and brain. J Biol Chem. 1995;270:27742–27751. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
