Activin potentiates proliferation in mature avian auditory sensory epithelium
- PMID: 20071511
- PMCID: PMC2975606
- DOI: 10.1523/JNEUROSCI.5154-09.2010
Activin potentiates proliferation in mature avian auditory sensory epithelium
Abstract
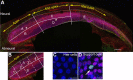
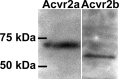
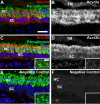
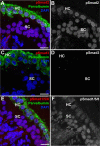
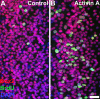
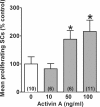
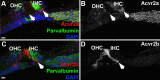
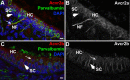
Humans and other mammals are highly susceptible to permanent hearing and balance deficits due to an inability to regenerate sensory hair cells lost to inner ear trauma. In contrast, nonmammalian vertebrates, such as birds, robustly regenerate replacement hair cells and restore hearing and balance functions to near-normal levels. There is considerable interest in understanding the cellular mechanisms responsible for this difference in regenerative capacity. Here we report on involvement of the TGFbeta superfamily type II activin receptors, Acvr2a and Acvr2b, in regulating proliferation in mature avian auditory sensory epithelium. Cultured, posthatch avian auditory sensory epithelium treated with Acvr2a and Acvr2b inhibitors shows decreased proliferation of support cells, the cell type that gives rise to new hair cells. Conversely, addition of activin A, an Acvr2a/b ligand, potentiates support cell proliferation. Neither treatment (inhibitor or ligand) affected hair cell survival, suggesting a specific effect of Acvr2a/b signaling on support cell mitogenicity. Using immunocytochemistry, Acvr2a, Acvr2b, and downstream Smad effector proteins were differentially localized in avian and mammalian auditory sensory epithelia. Collectively, these data suggest that signaling through Acvr2a/b promotes support cell proliferation in mature avian auditory sensory epithelium and that this signaling pathway may be incomplete, or actively blocked, in the adult mammalian ear.
Figures





Similar articles
-
Hair cell regeneration in the avian auditory epithelium.Int J Dev Biol. 2007;51(6-7):633-47. doi: 10.1387/ijdb.072408js. Int J Dev Biol. 2007. PMID: 17891722 Review.
-
Bone morphogenetic protein 4 antagonizes hair cell regeneration in the avian auditory epithelium.Hear Res. 2018 Jul;364:1-11. doi: 10.1016/j.heares.2018.04.008. Epub 2018 May 2. Hear Res. 2018. PMID: 29754876 Free PMC article.
-
Vascular endothelial growth factor is required for regeneration of auditory hair cells in the avian inner ear.Hear Res. 2020 Jan;385:107839. doi: 10.1016/j.heares.2019.107839. Epub 2019 Nov 8. Hear Res. 2020. PMID: 31760261
-
Regulation of p27Kip1 by Sox2 maintains quiescence of inner pillar cells in the murine auditory sensory epithelium.J Neurosci. 2012 Aug 1;32(31):10530-40. doi: 10.1523/JNEUROSCI.0686-12.2012. J Neurosci. 2012. PMID: 22855803 Free PMC article.
-
Sculpting the labyrinth: Morphogenesis of the developing inner ear.Semin Cell Dev Biol. 2017 May;65:47-59. doi: 10.1016/j.semcdb.2016.09.015. Epub 2016 Sep 26. Semin Cell Dev Biol. 2017. PMID: 27686400 Review.
Cited by
-
Growth hormone promotes hair cell regeneration in the zebrafish (Danio rerio) inner ear following acoustic trauma.PLoS One. 2011;6(11):e28372. doi: 10.1371/journal.pone.0028372. Epub 2011 Nov 30. PLoS One. 2011. PMID: 22140580 Free PMC article.
-
Norrie disease protein is essential for cochlear hair cell maturation.Proc Natl Acad Sci U S A. 2021 Sep 28;118(39):e2106369118. doi: 10.1073/pnas.2106369118. Proc Natl Acad Sci U S A. 2021. PMID: 34544869 Free PMC article.
-
A counter gradient of Activin A and follistatin instructs the timing of hair cell differentiation in the murine cochlea.Elife. 2019 Jun 12;8:e47613. doi: 10.7554/eLife.47613. Elife. 2019. PMID: 31187730 Free PMC article.
-
EGFR signaling is required for regenerative proliferation in the cochlea: conservation in birds and mammals.Dev Biol. 2012 Mar 1;363(1):191-200. doi: 10.1016/j.ydbio.2011.12.035. Epub 2012 Jan 2. Dev Biol. 2012. PMID: 22230616 Free PMC article.
-
A miR-590/Acvr2a/Rad51b axis regulates DNA damage repair during mESC proliferation.Stem Cell Reports. 2014 Dec 9;3(6):1103-17. doi: 10.1016/j.stemcr.2014.10.006. Epub 2014 Nov 20. Stem Cell Reports. 2014. PMID: 25458897 Free PMC article.
References
-
- Albertson RC, Payne-Ferreira TL, Postlethwait J, Yelick PC. Zebrafish acvr2a and acvr2b exhibit distinct roles in craniofacial development. Dev Dyn. 2005;233:1405–1418. - PubMed
-
- Bermingham-McDonogh O, Rubel EW. Hair cell regeneration: winging our way towards a sound future. Curr Opin Neurobiol. 2003;13:119–126. - PubMed
-
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. - PubMed
-
- Chang C, Harland RM. Neural induction requires continued suppression of both Smad1 and Smad2 signals during gastrulation. Development. 2007;134:3861–3872. - PubMed
-
- Chardin S, Romand R. Regeneration and mammalian auditory hair cells. Science. 1995;267:707–711. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
