Adaptation of reward sensitivity in orbitofrontal neurons
- PMID: 20071516
- PMCID: PMC2880492
- DOI: 10.1523/JNEUROSCI.4009-09.2010
Adaptation of reward sensitivity in orbitofrontal neurons
Abstract
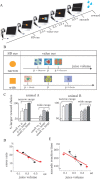
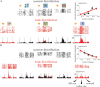
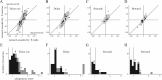
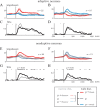
Animals depend on a large variety of rewards but their brains have a limited dynamic coding range. When rewards are uncertain, neuronal coding needs to cover a wide range of possible rewards. However, when reward is likely to occur within a specific range, focusing the sensitivity on the predicted range would optimize the discrimination of small reward differences. One way to overcome the trade-off between wide coverage and optimal discrimination is to adapt reward sensitivity dynamically to the available rewards. We investigated how changes in reward distribution influenced the coding of reward in the orbitofrontal cortex. Animals performed an oculomotor task in which a fixation cue predicted the SD of the probability distribution of juice volumes, while the expected mean volume was kept constant. A subsequent cue specified the exact juice volume obtained for a correct saccade response. Population responses of orbitofrontal neurons that reflected the predicted juice volume showed adaptation to the reward distribution. Statistical tests on individual responses revealed that a quarter of value-coding neurons shifted the reward sensitivity slope significantly between two reward distributions, whereas the remaining neurons showed insignificant change or lack of adaptation. Adaptations became more prominent when reward distributions changed less frequently, indicating time constraints for assessing reward distributions and adjusting neuronal sensitivity. The observed neuronal adaptation would optimize discrimination and contribute to the efficient coding of a large variety of potential rewards by neurons with limited dynamic range.
Figures



References
-
- Barlow H. Possible principles underlying the transformation of sensory messages. In: Rosenblith WA, editor. Sensory communication. Cambridge, MA: MIT; 1961. pp. 217–234.
-
- Baylis LL, Gaffan D. Amygdalectomy and ventromedial prefrontal ablation produce similar deficits in food choice and in simple object discrimination learning for an unseen reward. Exp Brain Res. 1991;86:617–622. - PubMed
-
- Brenner N, Bialek W, de Ruyter van Steveninck R. Adaptive rescaling maximizes information transmission. Neuron. 2000;26:695–702. - PubMed
-
- Cromwell HC, Hassani OK, Schultz W. Relative reward processing in primate striatum. Exp Brain Res. 2005;162:520–525. - PubMed
-
- Dean I, Harper NS, McAlpine D. Neural population coding of sound level adapts to stimulus statistics. Nat Neurosci. 2005;8:1684–1689. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
