Control of cannabinoid CB1 receptor function on glutamate axon terminals by endogenous adenosine acting at A1 receptors
- PMID: 20071517
- PMCID: PMC2855550
- DOI: 10.1523/JNEUROSCI.4920-09.2010
Control of cannabinoid CB1 receptor function on glutamate axon terminals by endogenous adenosine acting at A1 receptors
Abstract
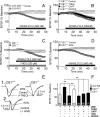
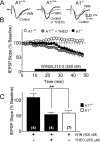
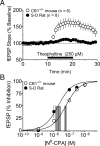
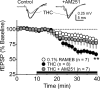
Marijuana is a widely used drug that impairs memory through interaction between its psychoactive constituent, Delta-9-tetrahydrocannabinol (Delta(9)-THC), and CB(1) receptors (CB1Rs) in the hippocampus. CB1Rs are located on Schaffer collateral (Sc) axon terminals in the hippocampus, where they inhibit glutamate release onto CA1 pyramidal neurons. This action is shared by adenosine A(1) receptors (A1Rs), which are also located on Sc terminals. Furthermore, A1Rs are tonically activated by endogenous adenosine (eADO), leading to suppressed glutamate release under basal conditions. Colocalization of A1Rs and CB1Rs, and their coupling to shared components of signal transduction, suggest that these receptors may interact. We examined the roles of A1Rs and eADO in regulating CB1R inhibition of glutamatergic synaptic transmission in the rodent hippocampus. We found that A1R activation by basal or experimentally increased levels of eADO reduced or eliminated CB1R inhibition of glutamate release, and that blockade of A1Rs with caffeine or other antagonists reversed this effect. The CB1R-A1R interaction was observed with the agonists WIN55,212-2 and Delta(9)-THC and during endocannabinoid-mediated depolarization-induced suppression of excitation. A1R control of CB1Rs was stronger in the C57BL/6J mouse hippocampus, in which eADO levels were higher than in Sprague Dawley rats, and the eADO modulation of CB1R effects was absent in A1R knock-out mice. Since eADO levels and A1R activation are regulated by homeostatic, metabolic, and pathological factors, these data identify a mechanism in which CB1R function can be controlled by the brain adenosine system. Additionally, our data imply that caffeine may potentiate the effects of marijuana on hippocampal function.
Figures


Similar articles
-
Species and strain differences in the expression of a novel glutamate-modulating cannabinoid receptor in the rodent hippocampus.Eur J Neurosci. 2005 Nov;22(9):2387-91. doi: 10.1111/j.1460-9568.2005.04401.x. Eur J Neurosci. 2005. PMID: 16262678 Free PMC article.
-
Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons.J Physiol. 2001 May 1;532(Pt 3):731-48. doi: 10.1111/j.1469-7793.2001.0731e.x. J Physiol. 2001. PMID: 11313442 Free PMC article.
-
Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus.Neuroscience. 2001;106(1):1-4. doi: 10.1016/s0306-4522(01)00287-1. Neuroscience. 2001. PMID: 11564411
-
The effects of cannabinoid 1 receptor compounds on memory: a meta-analysis and systematic review across species.Psychopharmacology (Berl). 2019 Nov;236(11):3257-3270. doi: 10.1007/s00213-019-05283-3. Epub 2019 Jun 5. Psychopharmacology (Berl). 2019. PMID: 31165913 Free PMC article.
-
Targeting corticostriatal transmission for the treatment of cannabinoid use disorder.Trends Pharmacol Sci. 2023 Aug;44(8):495-506. doi: 10.1016/j.tips.2023.05.003. Epub 2023 Jun 16. Trends Pharmacol Sci. 2023. PMID: 37331914 Free PMC article. Review.
Cited by
-
Review of the Endocannabinoid System.Biol Psychiatry Cogn Neurosci Neuroimaging. 2021 Jun;6(6):607-615. doi: 10.1016/j.bpsc.2020.07.016. Epub 2020 Aug 1. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021. PMID: 32980261 Free PMC article. Review.
-
Cannabinoid mitigation of neuronal morphological change important to development and learning: insight from a zebra finch model of psychopharmacology.Life Sci. 2013 Mar 19;92(8-9):467-75. doi: 10.1016/j.lfs.2012.07.031. Epub 2012 Aug 1. Life Sci. 2013. PMID: 22884809 Free PMC article. Review.
-
Cannabidiol Reverses Deficits in Hippocampal LTP in a Model of Alzheimer's Disease.Neurochem Res. 2019 Mar;44(3):703-713. doi: 10.1007/s11064-018-2513-z. Epub 2018 Mar 24. Neurochem Res. 2019. PMID: 29574668
-
Receptor mechanisms underlying the CNS effects of cannabinoids: CB1 receptor and beyond.Adv Pharmacol. 2022;93:275-333. doi: 10.1016/bs.apha.2021.10.006. Epub 2021 Dec 13. Adv Pharmacol. 2022. PMID: 35341569 Free PMC article.
-
Regulation of hippocampal cannabinoid CB1 receptor actions by adenosine A1 receptors and chronic caffeine administration: implications for the effects of Δ9-tetrahydrocannabinol on spatial memory.Neuropsychopharmacology. 2011 Jan;36(2):472-87. doi: 10.1038/npp.2010.179. Epub 2010 Oct 6. Neuropsychopharmacology. 2011. PMID: 20927050 Free PMC article.
References
-
- Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002;68:247–286. - PubMed
-
- Berman RF, Fredholm BB, Aden U, O'Connor WT. Evidence for increased dorsal hippocampal adenosine release and metabolism during pharmacologically induced seizures in rats. Brain Res. 2000;872:44–53. - PubMed
-
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
