Involvement of Ca2+ channel synprint site in synaptic vesicle endocytosis
- PMID: 20071530
- PMCID: PMC6632990
- DOI: 10.1523/JNEUROSCI.3214-09.2010
Involvement of Ca2+ channel synprint site in synaptic vesicle endocytosis
Abstract
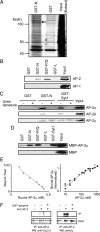
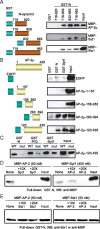
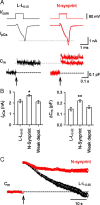
The synaptic protein interaction (synprint) site of the voltage-gated Ca(2+) channel (VGCC) alpha1 subunit can interact with proteins involved in exocytosis, and it is therefore thought to be essential for exocytosis of synaptic vesicles. Here we report that the synprint site can also directly bind the mu subunit of AP-2, an adaptor protein for clathrin-mediated endocytosis, in competition with the synaptotagmin 1 (Syt 1) C2B domain. In brain lysates, the AP-2-synprint interaction occurred over a wide range of Ca(2+) concentrations but was inhibited at high Ca(2+) concentrations, in which Syt 1 interacted with synprint site. At the calyx of Held synapse in rat brainstem slices, direct presynaptic loading of the synprint fragment peptide blocked endocytic, but not exocytic, membrane capacitance changes. We propose that the VGCC synprint site is involved in synaptic vesicle endocytosis, rather than exocytosis, in the nerve terminal, via Ca(2+)-dependent interactions with AP-2 and Syt.
Figures

Similar articles
-
Discrete residues in the c(2)b domain of synaptotagmin I independently specify endocytic rate and synaptic vesicle size.Neuron. 2006 Apr 6;50(1):49-62. doi: 10.1016/j.neuron.2006.02.021. Neuron. 2006. PMID: 16600855
-
Formation of an endophilin-Ca2+ channel complex is critical for clathrin-mediated synaptic vesicle endocytosis.Cell. 2003 Oct 3;115(1):37-48. doi: 10.1016/s0092-8674(03)00726-8. Cell. 2003. PMID: 14532001
-
Interaction of the synprint site of N-type Ca2+ channels with the C2B domain of synaptotagmin I.Proc Natl Acad Sci U S A. 1997 May 13;94(10):5405-10. doi: 10.1073/pnas.94.10.5405. Proc Natl Acad Sci U S A. 1997. PMID: 9144250 Free PMC article.
-
Physical link and functional coupling of presynaptic calcium channels and the synaptic vesicle docking/fusion machinery.J Bioenerg Biomembr. 1998 Aug;30(4):335-45. doi: 10.1023/a:1021985521748. J Bioenerg Biomembr. 1998. PMID: 9758330 Review.
-
Ca2+-dependent regulation of synaptic vesicle endocytosis.Neurosci Res. 2012 May;73(1):1-7. doi: 10.1016/j.neures.2012.02.012. Epub 2012 Mar 3. Neurosci Res. 2012. PMID: 22401840 Review.
Cited by
-
Nanoscale distribution of presynaptic Ca(2+) channels and its impact on vesicular release during development.Neuron. 2015 Jan 7;85(1):145-158. doi: 10.1016/j.neuron.2014.11.019. Epub 2014 Dec 18. Neuron. 2015. PMID: 25533484 Free PMC article.
-
Dual regulation of diacylglycerol kinase (DGK)-θ: polybasic proteins promote activation by phospholipids and increase substrate affinity.J Biol Chem. 2012 Dec 7;287(50):41619-27. doi: 10.1074/jbc.M112.404855. Epub 2012 Oct 22. J Biol Chem. 2012. PMID: 23091060 Free PMC article.
-
Control of neuronal voltage-gated calcium ion channels from RNA to protein.Trends Neurosci. 2013 Oct;36(10):598-609. doi: 10.1016/j.tins.2013.06.008. Epub 2013 Jul 30. Trends Neurosci. 2013. PMID: 23907011 Free PMC article. Review.
-
Human Pluripotent Stem Cell-Derived Neurons Are Functionally Mature In Vitro and Integrate into the Mouse Striatum Following Transplantation.Mol Neurobiol. 2020 Jun;57(6):2766-2798. doi: 10.1007/s12035-020-01907-4. Epub 2020 Apr 30. Mol Neurobiol. 2020. PMID: 32356172 Free PMC article.
-
Activity-dependent modulation of endocytosis by calmodulin at a large central synapse.Proc Natl Acad Sci U S A. 2012 Jan 3;109(1):291-6. doi: 10.1073/pnas.1100608109. Epub 2011 Dec 19. Proc Natl Acad Sci U S A. 2012. PMID: 22184217 Free PMC article.
References
-
- Borst JG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1996;383:431–434. - PubMed
-
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. - PubMed
-
- Chapman ER, Desai RC, Davis AF, Tornehl CK. Delineation of the oligomerization, AP-2 binding, and synprint binding region of the C2B domain of synaptotagmin. J Biol Chem. 1998;273:32966–32972. - PubMed
-
- Chen Y, Deng L, Maeno-Hikichi Y, Lai M, Chang S, Chen G, Zhang JF. Formation of an endophillin-Ca2+ channel complex is critical for clathrin-mediated synaptic vesicle endocytosis. Cell. 2003;115:37–48. - PubMed
-
- Diril MK, Wienisch M, Jung N, Klingauf J, Haucke V. Stonin 2 is an AP-2-dependent endocytic sorting adaptor for synaptotagmin internalization and recycling. Dev Cell. 2006;10:233–244. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
