Dysregulation of mTOR signaling in fragile X syndrome
- PMID: 20071534
- PMCID: PMC3665010
- DOI: 10.1523/JNEUROSCI.3696-09.2010
Dysregulation of mTOR signaling in fragile X syndrome
Abstract
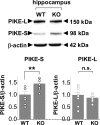
Fragile X syndrome, the most common form of inherited mental retardation and leading genetic cause of autism, is caused by transcriptional silencing of the Fmr1 gene. The fragile X mental retardation protein (FMRP), the gene product of Fmr1, is an RNA binding protein that negatively regulates translation in neurons. The Fmr1 knock-out mouse, a model of fragile X syndrome, exhibits cognitive deficits and exaggerated metabotropic glutamate receptor (mGluR)-dependent long-term depression at CA1 synapses. However, the molecular mechanisms that link loss of function of FMRP to aberrant synaptic plasticity remain unclear. The mammalian target of rapamycin (mTOR) signaling cascade controls initiation of cap-dependent translation and is under control of mGluRs. Here we show that mTOR phosphorylation and activity are elevated in hippocampus of juvenile Fmr1 knock-out mice by four functional readouts: (1) association of mTOR with regulatory associated protein of mTOR; (2) mTOR kinase activity; (3) phosphorylation of mTOR downstream targets S6 kinase and 4E-binding protein; and (4) formation of eukaryotic initiation factor complex 4F, a critical first step in cap-dependent translation. Consistent with this, mGluR long-term depression at CA1 synapses of FMRP-deficient mice is exaggerated and rapamycin insensitive. We further show that the p110 subunit of the upstream kinase phosphatidylinositol 3-kinase (PI3K) and its upstream activator PI3K enhancer PIKE, predicted targets of FMRP, are upregulated in knock-out mice. Elevated mTOR signaling may provide a functional link between overactivation of group I mGluRs and aberrant synaptic plasticity in the fragile X mouse, mechanisms relevant to impaired cognition in fragile X syndrome.
Figures

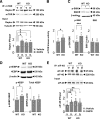
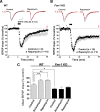
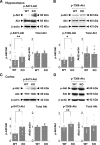
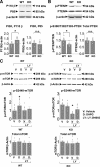
Comment in
-
Fragile X mental retardation protein: regulator of specific mRNAs or master regulator of global translation?J Neurosci. 2010 May 26;30(21):7121-3. doi: 10.1523/JNEUROSCI.1298-10.2010. J Neurosci. 2010. PMID: 20505079 Free PMC article. No abstract available.
Similar articles
-
Evidence for a fragile X mental retardation protein-mediated translational switch in metabotropic glutamate receptor-triggered Arc translation and long-term depression.J Neurosci. 2012 Apr 25;32(17):5924-36. doi: 10.1523/JNEUROSCI.4650-11.2012. J Neurosci. 2012. PMID: 22539853 Free PMC article.
-
Excess phosphoinositide 3-kinase subunit synthesis and activity as a novel therapeutic target in fragile X syndrome.J Neurosci. 2010 Aug 11;30(32):10624-38. doi: 10.1523/JNEUROSCI.0402-10.2010. J Neurosci. 2010. PMID: 20702695 Free PMC article.
-
Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile X syndrome.J Neurophysiol. 2006 May;95(5):3291-5. doi: 10.1152/jn.01316.2005. Epub 2006 Feb 1. J Neurophysiol. 2006. PMID: 16452252
-
Metabotropic glutamate receptors and fragile x mental retardation protein: partners in translational regulation at the synapse.Sci Signal. 2008 Feb 5;1(5):pe6. doi: 10.1126/stke.15pe6. Sci Signal. 2008. PMID: 18272470 Review.
-
Hippocampal dysfunction and cognitive impairment in Fragile-X Syndrome.Neurosci Biobehav Rev. 2016 Sep;68:563-574. doi: 10.1016/j.neubiorev.2016.06.033. Epub 2016 Jun 23. Neurosci Biobehav Rev. 2016. PMID: 27345143 Review.
Cited by
-
Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders.Curr Mol Med. 2015;15(2):146-67. doi: 10.2174/1566524015666150303003028. Curr Mol Med. 2015. PMID: 25732149 Free PMC article. Review.
-
Chronic pharmacological mGlu5 inhibition corrects fragile X in adult mice.Neuron. 2012 Apr 12;74(1):49-56. doi: 10.1016/j.neuron.2012.03.009. Neuron. 2012. PMID: 22500629 Free PMC article.
-
Dysregulation of FMRP/mTOR Signaling Cascade in Hypoxic-Ischemic Injury of Premature Human Brain.J Child Neurol. 2016 Mar;31(4):426-32. doi: 10.1177/0883073815596617. Epub 2015 Aug 3. J Child Neurol. 2016. PMID: 26239490 Free PMC article.
-
NMDA receptor activation regulates sociability by its effect on mTOR signaling activity.Prog Neuropsychopharmacol Biol Psychiatry. 2015 Jul 3;60:60-5. doi: 10.1016/j.pnpbp.2015.02.009. Epub 2015 Feb 20. Prog Neuropsychopharmacol Biol Psychiatry. 2015. PMID: 25703582 Free PMC article. Review.
-
Modeling fragile X syndrome in the Fmr1 knockout mouse.Intractable Rare Dis Res. 2014 Nov;3(4):118-33. doi: 10.5582/irdr.2014.01024. Intractable Rare Dis Res. 2014. PMID: 25606362 Free PMC article. Review.
References
-
- Bagni C, Greenough WT. From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat Rev Neurosci. 2005;6:376–387. - PubMed
-
- Brown V, Jin P, Ceman S, Darnell JC, O'Donnell WT, Tenenbaum SA, Jin X, Feng Y, Wilkinson KD, Keene JD, Darnell RB, Warren ST. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107:477–487. - PubMed
-
- Butler MG, Dasouki MJ, Zhou XP, Talebizadeh Z, Brown M, Takahashi TN, Miles JH, Wang CH, Stratton R, Pilarski R, Eng C. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J Med Genet. 2005;42:318–321. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
