Sox2 induces neuronal formation in the developing mammalian cochlea
- PMID: 20071536
- PMCID: PMC2835399
- DOI: 10.1523/JNEUROSCI.3852-09.2010
Sox2 induces neuronal formation in the developing mammalian cochlea
Abstract
In the cochlea, spiral ganglion neurons play a critical role in hearing as they form the relay between mechanosensory hair cells in the inner ear and cochlear nuclei in the brainstem. The proneural basic helix-loop-helix transcription factors Neurogenin1 (Neurog1) and NeuroD1 have been shown to be essential for the development of otocyst-derived inner ear sensory neurons. Here, we show neural competence of nonsensory epithelial cells in the cochlea, as ectopic expression of either Neurog1 or NeuroD1 results in the formation of neuronal cells. Since the high-mobility-group type transcription factor Sox2, which is also known to play a role in neurogenesis, is expressed in otocyst-derived neural precursor cells and later in the spiral ganglion neurons along with Neurog1 and NeuroD1, we used both gain- and loss-of-function experiments to examine the role of Sox2 in spiral ganglion neuron formation. We demonstrate that overexpression of Sox2 results in the production of neurons, suggesting that Sox2 is sufficient for the induction of neuronal fate in nonsensory epithelial cells. Furthermore, spiral ganglion neurons are absent in cochleae from Sox2(Lcc/Lcc) mice, indicating that Sox2 is also required for neuronal formation in the cochlea. Our results indicate that Sox2, along with Neurog1 and NeuroD1, are sufficient to induce a neuronal fate in nonsensory regions of the cochlea. Finally, we demonstrate that nonsensory cells within the cochlea retain neural competence through at least the early postnatal period.
Figures
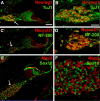
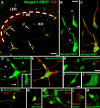

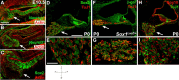



References
-
- Bylund M, Andersson E, Novitch BG, Muhr J. Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat Neurosci. 2003;6:1162–1168. - PubMed
-
- Chu K, Nemoz-Gaillard E, Tsai MJ. BETA2 and pancreatic islet development. Recent Prog Horm Res. 2001;56:23–46. - PubMed
-
- Collignon J, Sockanathan S, Hacker A, Cohen-Tannoudji M, Norris D, Rastan S, Stevanovic M, Goodfellow PN, Lovell-Badge R. A comparison of the properties of Sox-3 with Sry and two related genes, Sox-1 and Sox-2. Development. 1996;122:509–520. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
