GABAB receptor activation protects neurons from apoptosis via IGF-1 receptor transactivation
- PMID: 20071540
- PMCID: PMC6633015
- DOI: 10.1523/JNEUROSCI.2343-09.2010
GABAB receptor activation protects neurons from apoptosis via IGF-1 receptor transactivation
Erratum in
-
Erratum: Tu et al., "GABAB Receptor Activation Protects Neurons from Apoptosis via IGF-1 Receptor Transactivation".J Neurosci. 2024 Apr 24;44(17):e0548242024. doi: 10.1523/JNEUROSCI.0548-24.2024. J Neurosci. 2024. PMID: 38621998 Free PMC article. No abstract available.
Abstract
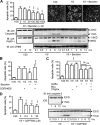
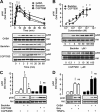
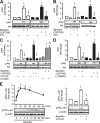
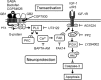
The G-protein-coupled receptors (GPCRs) and receptor tyrosine kinases (RTKs) play key roles in cell-cell communication. Several studies revealed important synergisms between these two types of receptors, with some of the actions of either receptor being mediated through transactivation of the other. Among the large GPCR family, GABA(B) receptor is activated by the neurotransmitter GABA, and is expressed in most neurons where it mediates slow and prolonged inhibition of synaptic transmission. Here we show that this receptor is involved in the regulation of life and death decisions of cerebellar granule neurons (CGNs). We show that specific activation of GABA(B) receptor can protect neurons from apoptosis through a mechanism that involves transactivation of the IGF-1 receptor (IGF-1R). Further work demonstrated that this cross talk was dependent on G(i/o)-protein, PLC, cytosolic Ca(2+), and FAK1 but independent of PKC, while IGF-1R-induced signaling involved Src kinase, PI3 kinase, and Akt activation. These results reveal a new function for this important GPCR and further highlight the importance of functional cross-talk networks between GPCRs and RTKs. Our results reveal GABA(B) receptor as a potential drug target for the treatment of neurodegenerative disorders.
Figures




Similar articles
-
Regulation of synaptic input to hypothalamic presympathetic neurons by GABA(B) receptors.Neuroscience. 2006 Oct 13;142(2):595-606. doi: 10.1016/j.neuroscience.2006.06.039. Epub 2006 Aug 2. Neuroscience. 2006. PMID: 16887273
-
P2X7 nucleotide receptor is coupled to GSK-3 inhibition and neuroprotection in cerebellar granule neurons.Neurotox Res. 2009 Apr;15(3):193-204. doi: 10.1007/s12640-009-9020-6. Epub 2009 Feb 24. Neurotox Res. 2009. PMID: 19384592
-
Mechanism of GABA receptor-mediated inhibition of spontaneous GABA release onto cerebellar Purkinje cells.Eur J Neurosci. 2004 Aug;20(3):684-700. doi: 10.1111/j.1460-9568.2004.03505.x. Eur J Neurosci. 2004. PMID: 15255979
-
GABAB receptor-mediated inhibition of GABAA receptor calcium elevations in developing hypothalamic neurons.J Neurophysiol. 1998 Mar;79(3):1360-70. doi: 10.1152/jn.1998.79.3.1360. J Neurophysiol. 1998. PMID: 9497417
-
Pharmacologic and therapeutic aspects of the developmentally regulated expression of GABA(A) and GABA(B) receptors: cerebellar granule cells as a model system.Neurochem Int. 1999 May;34(5):373-7. doi: 10.1016/s0197-0186(99)00044-3. Neurochem Int. 1999. PMID: 10397364 Review.
Cited by
-
Oligomeric Receptor Complexes and Their Allosteric Receptor-Receptor Interactions in the Plasma Membrane Represent a New Biological Principle for Integration of Signals in the CNS.Front Mol Neurosci. 2019 Sep 25;12:230. doi: 10.3389/fnmol.2019.00230. eCollection 2019. Front Mol Neurosci. 2019. PMID: 31607863 Free PMC article.
-
New Insights into the Neuromyogenic Spectrum of a Gain of Function Mutation in SPTLC1.Genes (Basel). 2022 May 17;13(5):893. doi: 10.3390/genes13050893. Genes (Basel). 2022. PMID: 35627278 Free PMC article.
-
Neuroendocrine-derived peptides promote prostate cancer cell survival through activation of IGF-1R signaling.Prostate. 2013 Jun;73(8):801-12. doi: 10.1002/pros.22624. Epub 2012 Nov 28. Prostate. 2013. PMID: 23192379 Free PMC article.
-
Intragenic deletions of the IGF1 receptor gene in five individuals with psychiatric phenotypes and developmental delay.Eur J Hum Genet. 2013 Nov;21(11):1304-7. doi: 10.1038/ejhg.2013.42. Epub 2013 Mar 13. Eur J Hum Genet. 2013. PMID: 23486542 Free PMC article.
-
Dysregulation of IGF-1/GLP-1 signaling in the progression of ALS: potential target activators and influences on neurological dysfunctions.Neurol Sci. 2021 Aug;42(8):3145-3166. doi: 10.1007/s10072-021-05328-6. Epub 2021 May 21. Neurol Sci. 2021. PMID: 34018075 Review.
References
-
- Andersson S, D'Arcy P, Larsson O, Sehat B. Focal adhesion kinase (FAK) activates and stabilizes IGF-1 receptor. Biochem Biophys Res Commun. 2009;387:36–41. - PubMed
-
- Belcheva MM, Haas PD, Tan Y, Heaton VM, Coscia CJ. The fibroblast growth factor receptor is at the site of convergence between mu-opioid receptor and growth factor signaling pathways in rat C6 glioma cells. J Pharmacol Exp Ther. 2002;303:909–918. - PubMed
-
- Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–1284. - PubMed
-
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signaling. Nat Rev Mol Cell Biol. 2000;1:11–21. - PubMed
-
- Bettler B, Kaupmann K, Bowery N. GABAB receptors: drugs meet clones. Curr Opin Neurobiol. 1998;8:345–350. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
