Temporal codes for amplitude contrast in auditory cortex
- PMID: 20071542
- PMCID: PMC3551278
- DOI: 10.1523/JNEUROSCI.4170-09.2010
Temporal codes for amplitude contrast in auditory cortex
Abstract
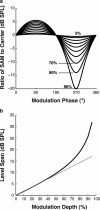
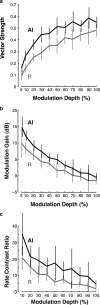
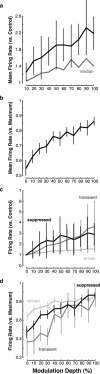
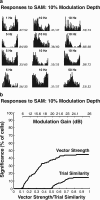
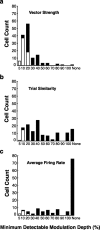
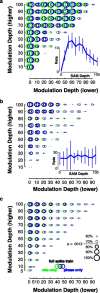
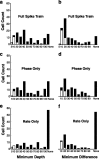
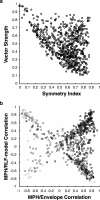
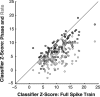
The encoding of sound level is fundamental to auditory signal processing, and the temporal information present in amplitude modulation is crucial to the complex signals used for communication sounds, including human speech. The modulation transfer function, which measures the minimum detectable modulation depth across modulation frequency, has been shown to predict speech intelligibility performance in a range of adverse listening conditions and hearing impairments, and even for users of cochlear implants. We presented sinusoidal amplitude modulation (SAM) tones of varying modulation depths to awake macaque monkeys while measuring the responses of neurons in the auditory core. Using spike train classification methods, we found that thresholds for modulation depth detection and discrimination in the most sensitive units are comparable to psychophysical thresholds when precise temporal discharge patterns rather than average firing rates are considered. Moreover, spike timing information was also superior to average rate information when discriminating static pure tones varying in level but with similar envelopes. The limited utility of average firing rate information in many units also limited the utility of standard measures of sound level tuning, such as the rate level function (RLF), in predicting cortical responses to dynamic signals like SAM. Response modulation typically exceeded that predicted by the slope of the RLF by large factors. The decoupling of the cortical encoding of SAM and static tones indicates that enhancing the representation of acoustic contrast is a cardinal feature of the ascending auditory pathway.
Figures

References
-
- Bieser A, Müller-Preuss P. Auditory responsive cortex in the squirrel monkey: neural responses to amplitude-modulated sounds. Exp Brain Res. 1996;108:273–284. - PubMed
-
- Busby PA, Tong YC, Clark GM. The perception of temporal modulations by cochlear implant patients. J Acoust Soc Am. 1993;94:124–131. - PubMed
-
- Cazals Y, Pelizzone M, Saudan O, Boex C. Low-pass filtering in amplitude modulation detection associated with vowel and consonant identification in subjects with cochlear implants. J Acoust Soc Am. 1994;96:2048–2054. - PubMed
-
- Cohen YE, Theunissen F, Russ BE, Gill P. Acoustic features of rhesus vocalizations and their representation in the ventrolateral prefrontal cortex. J Neurophysiol. 2007;97:1470–1484. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
