Development of a reverse transcription-loop-mediated isothermal amplification assay for detection of pandemic (H1N1) 2009 virus as a novel molecular method for diagnosis of pandemic influenza in resource-limited settings
- PMID: 20071551
- PMCID: PMC2832456
- DOI: 10.1128/JCM.01481-09
Development of a reverse transcription-loop-mediated isothermal amplification assay for detection of pandemic (H1N1) 2009 virus as a novel molecular method for diagnosis of pandemic influenza in resource-limited settings
Abstract
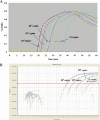
This paper reports on the development of a one-step, real-time reverse transcription-loop-mediated isothermal amplification (RT-LAMP) assay targeting the hemagglutinin (HA) gene for the rapid molecular-based detection of pandemic (H1N1) 2009 virus. The detection limit of the pandemic (H1N1) 2009 virus HA-specific RT-LAMP assay was same as that of the currently used real-time reverse transcription-PCR method. The assay detected the pandemic (H1N1) 2009 virus HA gene in 136 RNA samples extracted from nasopharyngeal swab specimens from Japanese and Vietnamese patients. No cross-reactive amplification with the RNA of other seasonal influenza viruses was observed, and the detection of specific viral genome targets in clinical specimens was achieved in less than 40 min. The sensitivity and specificity of the pandemic (H1N1) 2009 virus HA-specific RT-LAMP assay obtained in this study were 97.8% and 100%, respectively. Use of the (H1N1) 2009 virus HA-specific RT-LAMP assay will enable the faster and easier diagnosis of pandemic (H1N1) 2009 virus infection, especially in resource-limited situations in developing countries.
Figures

Similar articles
-
Evaluation of reverse transcription loop-mediated isothermal amplification assays for rapid diagnosis of pandemic influenza A/H1N1 2009 virus.J Med Virol. 2011 Jan;83(1):10-5. doi: 10.1002/jmv.21934. J Med Virol. 2011. PMID: 21108334
-
[Colorimetric detection of human influenza A H1N1 virus by reverse transcription loop mediated isothermal amplification].Bing Du Xue Bao. 2010 Mar;26(2):81-7. Bing Du Xue Bao. 2010. PMID: 20480635 Chinese.
-
One-step detection of the 2009 pandemic influenza A(H1N1) virus by the RT-SmartAmp assay and its clinical validation.PLoS One. 2012;7(1):e30236. doi: 10.1371/journal.pone.0030236. Epub 2012 Jan 25. PLoS One. 2012. PMID: 22295077 Free PMC article.
-
Tools to detect influenza virus.Yonsei Med J. 2013 May 1;54(3):560-6. doi: 10.3349/ymj.2013.54.3.560. Yonsei Med J. 2013. PMID: 23549796 Free PMC article. Review.
-
Comparison of Respiratory Specimen Collection Methods for Detection of Influenza Virus Infection by Reverse Transcription-PCR: a Literature Review.J Clin Microbiol. 2019 Aug 26;57(9):e00027-19. doi: 10.1128/JCM.00027-19. Print 2019 Sep. J Clin Microbiol. 2019. PMID: 31217267 Free PMC article.
Cited by
-
LAMPPrimerBank, a manually curated database of experimentally validated loop-mediated isothermal amplification primers for detection of respiratory pathogens.Infection. 2023 Dec;51(6):1809-1818. doi: 10.1007/s15010-023-02100-0. Epub 2023 Oct 12. Infection. 2023. PMID: 37828369
-
Nucleic Acid-Based Sensing Techniques for Diagnostics and Surveillance of Influenza.Biosensors (Basel). 2021 Feb 12;11(2):47. doi: 10.3390/bios11020047. Biosensors (Basel). 2021. PMID: 33673035 Free PMC article. Review.
-
Specific Detection of Influenza A and B Viruses by CRISPR-Cas12a-Based Assay.Biosensors (Basel). 2021 Mar 19;11(3):88. doi: 10.3390/bios11030088. Biosensors (Basel). 2021. PMID: 33808752 Free PMC article.
-
Two years after pandemic influenza A/2009/H1N1: what have we learned?Clin Microbiol Rev. 2012 Apr;25(2):223-63. doi: 10.1128/CMR.05012-11. Clin Microbiol Rev. 2012. PMID: 22491771 Free PMC article. Review.
-
Recent advances in the detection of respiratory virus infection in humans.J Med Virol. 2020 Apr;92(4):408-417. doi: 10.1002/jmv.25674. Epub 2020 Feb 4. J Med Virol. 2020. PMID: 31944312 Free PMC article. Review.
References
-
- Anonymous. 2009. Swine influenza A (H1N1) infection in two children—Southern California, March-April 2009. MMWR Morb. Mortal. Wkly. Rep. 58:400-402. - PubMed
-
- Carr, M. J., R. Gunson, A. Maclean, S. Coughlan, M. Fitzgerald, M. Scully, B. O'Herlihy, J. Ryan, D. O'Flanagan, J. Connell, W. F. Carman, and W. W. Hall. 2009. Development of a real-time RT-PCR for the detection of swine-lineage influenza A (H1N1) virus infections. J. Clin. Virol. 45:196-199. - PMC - PubMed
-
- Chan, K. H., S. T. Lai, L. L. Poon, Y. Guan, K. Y. Yuen, and J. S. Peiris. 2009. Analytical sensitivity of rapid influenza antigen detection tests for swine-origin influenza virus (H1N1). J. Clin. Virol. 45:205-207. - PubMed
-
- Dawood, F. S., S. Jain, L. Finelli, M. W. Shaw, S. Lindstrom, R. J. Garten, L. V. Gubareva, X. Xu, C. B. Bridges, and T. M. Uyeki. 2009. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N. Engl. J. Med. 360:2605-2615. - PubMed
-
- Faix, D. J., S. S. Sherman, and S. H. Waterman. 2009. Rapid-test sensitivity for novel swine-origin influenza A (H1N1) virus in humans. N. Engl. J. Med. 361:728-729. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

