Gut mucosal FOXP3+ regulatory CD4+ T cells and Nonregulatory CD4+ T cells are differentially affected by simian immunodeficiency virus infection in rhesus macaques
- PMID: 20071575
- PMCID: PMC2838127
- DOI: 10.1128/JVI.01715-09
Gut mucosal FOXP3+ regulatory CD4+ T cells and Nonregulatory CD4+ T cells are differentially affected by simian immunodeficiency virus infection in rhesus macaques
Abstract
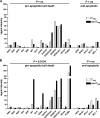
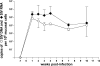
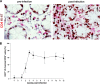
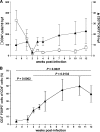
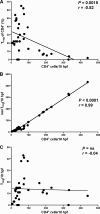
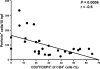
The gastrointestinal tract represents a major site for human and simian immunodeficiency virus (HIV and SIV) replication and CD4(+) T-cell depletion. Despite severe depletion of mucosal CD4(+) T cells, FOXP3(+) regulatory CD4(+) T cells (T(reg)) are highly increased in the gut mucosa of chronically HIV-infected individuals and may contribute to HIV pathogenesis, either by their immunosuppressive function or as a significant target cell population for virus production. Little is known about the susceptibility of mucosal T(reg) to viral infection and the longitudinal effect of HIV/SIV infection on T(reg) dynamics. In this study, we determined the level of SIV infection in T(reg) and nonregulatory CD4(+) T cells (non-T(reg)) isolated from the colon of SIV-infected rhesus macaques. The dynamics of mucosal T(reg) and alterations in the mucosal CD4(+) T-cell pool were examined longitudinally. Our findings indicate that mucosal T(reg) were less susceptible to productive SIV infection than non-T(reg) and thus were selectively spared from SIV-mediated cell death. In addition to improved survival, local expansion of T(reg) by SIV-induced proliferation of the mucosal CD4(+) T-cell pool facilitated the accumulation of mucosal T(reg) during the course of infection. High frequency of mucosal T(reg) in chronic SIV infection was strongly related to a reduction of perforin-expressing cells. In conclusion, this study suggests that mucosal T(reg) are less affected by productive SIV infection than non-T(reg) and therefore spared from depletion. Although SIV production is limited in mucosal T(reg), T(reg) accumulation may indirectly contribute to viral persistence by suppressing antiviral immune responses.
Figures



References
-
- Allers, K., D. Kunkel, V. Moos, M. Eisenblatter, C. Stahl-Hennig, F. J. Kaup, R. Ignatius, and T. Schneider. 2008. Migration patterns of nonspecifically activated versus nonactivated nonhuman primate T lymphocytes: preferential homing of activated autologous CD8+ T cells in the rectal mucosa. J. Immunother. 31:334-344. - PubMed
-
- Andersen, J. L., E. S. Zimmerman, J. L. DeHart, S. Murala, O. Ardon, J. Blackett, J. Chen, and V. Planelles. 2005. ATR and GADD45alpha mediate HIV-1 Vpr-induced apoptosis. Cell Death Differ. 12:326-334. - PubMed
-
- Andersson, J., H. Behbahani, J. Lieberman, E. Connick, A. Landay, B. Patterson, A. Sonnerborg, K. Lore, S. Uccini, and T. E. Fehniger. 1999. Perforin is not co-expressed with granzyme A within cytotoxic granules in CD8 T lymphocytes present in lymphoid tissue during chronic HIV infection. AIDS 13:1295-1303. - PubMed
-
- Andersson, J., S. Kinloch, A. Sonnerborg, J. Nilsson, T. E. Fehniger, A. L. Spetz, H. Behbahani, L. E. Goh, H. McDade, B. Gazzard, H. Stellbrink, D. Cooper, and L. Perrin. 2002. Low levels of perforin expression in CD8+ T lymphocyte granules in lymphoid tissue during acute human immunodeficiency virus type 1 infection. J. Infect. Dis. 185:1355-1358. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

