Preexisting infection with human T-cell lymphotropic virus type 2 neither exacerbates nor attenuates simian immunodeficiency virus SIVmac251 infection in macaques
- PMID: 20071587
- PMCID: PMC2826058
- DOI: 10.1128/JVI.01655-09
Preexisting infection with human T-cell lymphotropic virus type 2 neither exacerbates nor attenuates simian immunodeficiency virus SIVmac251 infection in macaques
Abstract
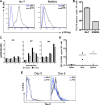
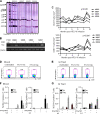
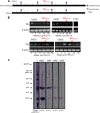
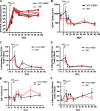
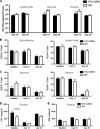
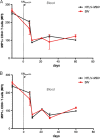
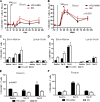
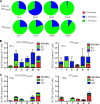
Coinfection with human T-cell lymphotropic virus type 2 (HTLV-2) and human immunodeficiency virus type 1 (HIV-1) has been reported to have either a slowed disease course or to have no effect on progression to AIDS. In this study, we generated a coinfection animal model and investigated whether HTLV-2 could persistently infect macaques, induce a T-cell response, and impact simian immunodeficiency virus SIV(mac251)-induced disease. We found that inoculation of irradiated HTLV-2-infected T cells into Indian rhesus macaques elicited humoral and T-cell responses to HTLV-2 antigens at both systemic and mucosal sites. Low levels of HTLV-2 provirus DNA were detected in the blood, lymphoid tissues, and gastrointestinal tracts of infected animals. Exposure of HTLV-2-infected or naïve macaques to SIV(mac251) demonstrated comparable levels of SIV(mac251) viral replication, similar rates of mucosal and peripheral CD4(+) T-cell loss, and increased T-cell proliferation. Additionally, neither the magnitude nor the functional capacity of the SIV-specific T-cell-mediated immune response was different in HTLV-2/SIV(mac251) coinfected animals versus SIV(mac251) singly infected controls. Thus, HTLV-2 targets mucosal sites, persists, and importantly does not exacerbate SIV(mac251) infection. These data provide the impetus for the development of an attenuated HTLV-2-based vectored vaccine for HIV-1; this approach could elicit persistent mucosal immunity that may prevent HIV-1/SIV(mac251) infection.
Figures
References
-
- Araujo, A., and W. W. Hall. 2004. Human T-lymphotropic virus type II and neurological disease. Ann. Neurol. 56:10-19. - PubMed
-
- Bartholomew, C., W. Blattner, and F. Cleghorn. 1987. Progression to AIDS in homosexual men co-infected with HIV and HTLV-I in Trinidad. Lancet 2:1469. - PubMed
-
- Bassani, S., M. Lopez, C. Toro, V. Jimenez, J. M. Sempere, V. Soriano, and J. M. Benito. 2007. Influence of human T cell lymphotropic virus type 2 coinfection on virological and immunological parameters in HIV type 1-infected patients. Clin. Infect. Dis. 44:105-110. - PubMed
-
- Beilke, M. A., K. P. Theall, M. O'Brien, J. L. Clayton, S. M. Benjamin, E. L. Winsor, and P. J. Kissinger. 2004. Clinical outcomes and disease progression among patients coinfected with HIV and human T lymphotropic virus types 1 and 2. Clin. Infect. Dis. 39:256-263. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

