Role of purinergic signaling pathways in V-ATPase recruitment to apical membrane of acidifying epididymal clear cells
- PMID: 20071692
- PMCID: PMC2853219
- DOI: 10.1152/ajpcell.00460.2009
Role of purinergic signaling pathways in V-ATPase recruitment to apical membrane of acidifying epididymal clear cells
Abstract
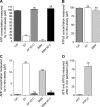
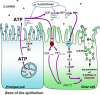
Extracellular purinergic agonists regulate a broad range of physiological functions via P1 and P2 receptors. Using the epididymis as a model system in which luminal acidification is essential for sperm maturation and storage, we show here that extracellular ATP and its hydrolysis product adenosine trigger the apical accumulation of vacuolar H(+)-ATPase (V-ATPase) in acidifying clear cells. We demonstrate that the epididymis can hydrolyze luminal ATP into other purinergic agonists such as ADP via the activity of nucleotidases located in the epididymal fluid and in the apical membrane of epithelial cells. Alkaline phosphatase activity and abundant ecto-5'-nucleotidase protein were detected in the apical pole of principal cells. In addition, we show that nine nucleotidase genes (Nt5e, Alpl, Alpp, Enpp1, 2, and 3, and Entpd 2, 4, and 5), seven ATP P2 receptor genes (P2X1, P2X2, P2X3, P2X4, P2X6, P2Y2, P2Y5), and three adenosine P1 receptor genes (A1, A2B, and A3) are expressed in epithelial cells isolated by laser cut microdissection (LCM). The calcium chelator BAPTA-AM abolished the apical V-ATPase accumulation induced by ATP, supporting the contribution of P2X or P2Y in this response. The PKA inhibitor myristoylated protein kinase inhibitor (mPKI) inhibited adenosine-dependent V-ATPase apical accumulation, indicating the participation of the P1 A2B receptor. Altogether, these results suggest that the activation of P1 and P2 purinergic receptors by ATP and adenosine might play a significant role in luminal acidification in the epididymis, a process that is crucial for the establishment of male fertility.
Figures







References
-
- Acott TS, Carr DW. Inhibition of bovine spermatozoa by caudal epididymal fluid. II. Interaction of pH and a quiescence factor. Biol Reprod 30: 926–935, 1984 - PubMed
-
- Beaulieu V, Da Silva N, Pastor-Soler N, Brown CR, Smith PJ, Brown D, Breton S. Modulation of the actin cytoskeleton via gelsolin regulates vacuolar H+-ATPase recycling. J Biol Chem 280: 8452–8463, 2005 - PubMed
-
- Biber J, Stieger B, Haase W, Murer H. A high yield preparation for rat kidney brush border membranes. Different behaviour of lysosomal markers. Biochim Biophys Acta 647: 169–176, 1981 - PubMed
-
- Breton S, Brown D. New insights into the regulation of V-ATPase-dependent proton secretion. Am J Physiol Renal Physiol 292: F1–F10, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

