Functional cooperation between CREM and GCNF directs gene expression in haploid male germ cells
- PMID: 20071744
- PMCID: PMC2853129
- DOI: 10.1093/nar/gkp1220
Functional cooperation between CREM and GCNF directs gene expression in haploid male germ cells
Abstract
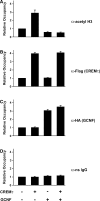
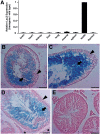
Cellular differentiation and development of germ cells critically depend on a coordinated activation and repression of specific genes. The underlying regulation mechanisms, however, still lack a lot of understanding. Here, we describe that both the testis-specific transcriptional activator CREMtau (cAMP response element modulator tau) and the repressor GCNF (germ cell nuclear factor) have an overlapping binding site which alone is sufficient to direct cell type-specific expression in vivo in a heterologous promoter context. Expression of the transgene driven by the CREM/GCNF site is detectable in spermatids, but not in any somatic tissue or at any other stages during germ cell differentiation. CREMtau acts as an activator of gene transcription whereas GCNF suppresses this activity. Both factors compete for binding to the same DNA response element. Effective binding of CREM and GCNF highly depends on composition and epigenetic modification of the binding site. We also discovered that CREM and GCNF bind to each other via their DNA binding domains, indicating a complex interaction between the two factors. There are several testis-specific target genes that are regulated by CREM and GCNF in a reciprocal manner, showing a similar activation pattern as during spermatogenesis. Our data indicate that a single common binding site for CREM and GCNF is sufficient to specifically direct gene transcription in a tissue-, cell type- and differentiation-specific manner.
Figures
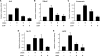
References
-
- Russell LD, Ettlin RA, Hikim A.PS, Clegg ED. Histological and Histopathological Evaluation of the Testis. Clearwater, FL, USA: Cache River Press; 1990. pp. 1–40.
-
- Cho C, Willis WD, Goulding EH, Jung-Ha H, Choi YC, Hecht NB, Eddy EM. Haploinsufficiency of protamine-1 or -2 causes infertility in mice. Nat. Genet. 2001;28:82–86. - PubMed
-
- Krege JH, John SW, Langenbach LL, Hodgin JB, Hagaman JR, Bachman ES, Jennette JC, O'B;rien DA, Smithies O. Male-female differences in fertility and blood pressure in ACE-deficient mice. Nature. 1995;375:146–148. - PubMed
-
- Nishimune Y, Tanaka H. Infertility caused by polymorphisms or mutations in spermatogenesis-specific genes. J. Androl. 2006;27:326–334. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

