Efficient transcription through an intron requires the binding of an Sm-type U1 snRNP with intact stem loop II to the splice donor
- PMID: 20071748
- PMCID: PMC2875018
- DOI: 10.1093/nar/gkp1224
Efficient transcription through an intron requires the binding of an Sm-type U1 snRNP with intact stem loop II to the splice donor
Abstract
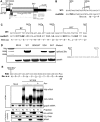
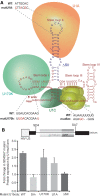
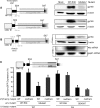
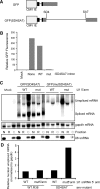
The mechanism behind the positive action of introns upon transcription and the biological significance of this positive feedback remains unclear. Functional ablation of splice sites within an HIV-derived env cDNA significantly reduced transcription that was rescued by a U1 snRNA modified to bind to the mutated splice donor (SD). Using this model we further characterized both the U1 and pre-mRNA structural requirements for transcriptional enhancement. U1 snRNA rescued as a mature Sm-type snRNP with an intact stem loop II. Position and sequence context for U1-binding is crucial because a promoter proximal intron placed upstream of the mutated SD failed to rescue transcription. Furthermore, U1-rescue was independent of promoter and exon sequence and is partially replaced by the transcription elongation activator Tat, pointing to an intron-localized block in transcriptional elongation. Thus, transcriptional coupling of U1 snRNA binding to the SD may licence the polymerase for transcription through the intron.
Figures


Similar articles
-
Crystal structure of human U1 snRNP, a small nuclear ribonucleoprotein particle, reveals the mechanism of 5' splice site recognition.Elife. 2015 Jan 2;4:e04986. doi: 10.7554/eLife.04986. Elife. 2015. PMID: 25555158 Free PMC article.
-
The upstream 5' splice site remains associated to the transcription machinery during intron synthesis.Nat Commun. 2021 Jul 27;12(1):4545. doi: 10.1038/s41467-021-24774-6. Nat Commun. 2021. PMID: 34315864 Free PMC article.
-
An intronic splicing enhancer binds U1 snRNPs to enhance splicing and select 5' splice sites.Mol Cell Biol. 2000 Dec;20(24):9225-35. doi: 10.1128/MCB.20.24.9225-9235.2000. Mol Cell Biol. 2000. PMID: 11094074 Free PMC article.
-
A novel role of U1 snRNP: Splice site selection from a distance.Biochim Biophys Acta Gene Regul Mech. 2019 Jun;1862(6):634-642. doi: 10.1016/j.bbagrm.2019.04.004. Epub 2019 Apr 28. Biochim Biophys Acta Gene Regul Mech. 2019. PMID: 31042550 Free PMC article. Review.
-
Can donor splice site recognition occur without the involvement of U1 snRNP?Biochem Soc Trans. 2008 Jun;36(Pt 3):548-50. doi: 10.1042/BST0360548. Biochem Soc Trans. 2008. PMID: 18482005 Review.
Cited by
-
Multiply spliced HIV RNA is a predictive measure of virus production ex vivo and in vivo following reversal of HIV latency.EBioMedicine. 2021 Mar;65:103241. doi: 10.1016/j.ebiom.2021.103241. Epub 2021 Feb 26. EBioMedicine. 2021. PMID: 33647768 Free PMC article.
-
Hyperimmune bovine colostrum as a low-cost, large-scale source of antibodies with broad neutralizing activity for HIV-1 envelope with potential use in microbicides.Antimicrob Agents Chemother. 2012 Aug;56(8):4310-9. doi: 10.1128/AAC.00453-12. Epub 2012 Jun 4. Antimicrob Agents Chemother. 2012. PMID: 22664963 Free PMC article.
-
Post-transcriptional spliceosomes are retained in nuclear speckles until splicing completion.Nat Commun. 2012;3:994. doi: 10.1038/ncomms1998. Nat Commun. 2012. PMID: 22871813
-
Therapeutic activity of modified U1 core spliceosomal particles.Nat Commun. 2016 Apr 4;7:11168. doi: 10.1038/ncomms11168. Nat Commun. 2016. PMID: 27041075 Free PMC article.
-
Excessive RNA splicing and inhibition of HIV-1 replication induced by modified U1 small nuclear RNAs.J Virol. 2010 Dec;84(24):12790-800. doi: 10.1128/JVI.01257-10. Epub 2010 Oct 6. J Virol. 2010. PMID: 20926575 Free PMC article.
References
-
- Moore MJ, Proudfoot NJ. Pre-mRNA processing reaches back to transcription and ahead to translation. Cell. 2009;136:688–700. - PubMed
-
- Chapman RD, Heidemann M, Hintermair C, Eick D. Molecular evolution of the RNA polymerase II CTD. Trends Genet. 2008;24:289–296. - PubMed
-
- Buratowski S. The CTD code. Nat. Struct. Biol. 2003;10:679–680. - PubMed
-
- Lu H, Zawel L, Fisher L, Egly JM, Reinberg D. Human general transcription factor IIH phosphorylates the C-terminal domain of RNA polymerase II. Nature. 1992;358:641–645. - PubMed
-
- Moteki S, Price D. Functional coupling of capping and transcription of mRNA. Mol. Cell. 2002;10:599–609. - PubMed

