Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease
- PMID: 20071773
- PMCID: PMC2810078
- DOI: 10.1172/JCI39733
Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease
Abstract
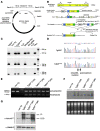
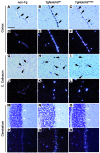
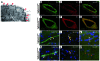
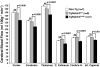
Cerebral ischemic small vessel disease (SVD) is the leading cause of vascular dementia and a major contributor to stroke in humans. Dominant mutations in NOTCH3 cause cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), a genetic archetype of cerebral ischemic SVD. Progress toward understanding the pathogenesis of this disease and developing effective therapies has been hampered by the lack of a good animal model. Here, we report the development of a mouse model for CADASIL via the introduction of a CADASIL-causing Notch3 point mutation into a large P1-derived artificial chromosome (PAC). In vivo expression of the mutated PAC transgene in the mouse reproduced the endogenous Notch3 expression pattern and main pathological features of CADASIL, including Notch3 extracellular domain aggregates and granular osmiophilic material (GOM) deposits in brain vessels, progressive white matter damage, and reduced cerebral blood flow. Mutant mice displayed attenuated myogenic responses and reduced caliber of brain arteries as well as impaired cerebrovascular autoregulation and functional hyperemia. Further, we identified a substantial reduction of white matter capillary density. These neuropathological changes occurred in the absence of either histologically detectable alterations in cerebral artery structure or blood-brain barrier breakdown. These studies provide in vivo evidence for cerebrovascular dysfunction and microcirculatory failure as key contributors to hypoperfusion and white matter damage in this genetic model of ischemic SVD.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

