Constitutively active phosphatase inhibitor-1 improves cardiac contractility in young mice but is deleterious after catecholaminergic stress and with aging
- PMID: 20071777
- PMCID: PMC2810086
- DOI: 10.1172/JCI40545
Constitutively active phosphatase inhibitor-1 improves cardiac contractility in young mice but is deleterious after catecholaminergic stress and with aging
Abstract
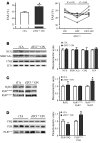
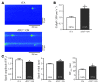
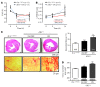
Phosphatase inhibitor-1 (I-1) is a distal amplifier element of beta-adrenergic signaling that functions by preventing dephosphorylation of downstream targets. I-1 is downregulated in human failing hearts, while overexpression of a constitutively active mutant form (I-1c) reverses contractile dysfunction in mouse failing hearts, suggesting that I-1c may be a candidate for gene therapy. We generated mice with conditional cardiomyocyte-restricted expression of I-1c (referred to herein as dTGI-1c mice) on an I-1-deficient background. Young adult dTGI-1c mice exhibited enhanced cardiac contractility but exaggerated contractile dysfunction and ventricular dilation upon catecholamine infusion. Telemetric ECG recordings revealed typical catecholamine-induced ventricular tachycardia and sudden death. Doxycycline feeding switched off expression of cardiomyocyte-restricted I-1c and reversed all abnormalities. Hearts from dTGI-1c mice showed hyperphosphorylation of phospholamban and the ryanodine receptor, and this was associated with an increased number of catecholamine-induced Ca2+ sparks in isolated myocytes. Aged dTGI-1c mice spontaneously developed a cardiomyopathic phenotype. These data were confirmed in a second independent transgenic mouse line, expressing a full-length I-1 mutant that could not be phosphorylated and thereby inactivated by PKC-alpha (I-1S67A). In conclusion, conditional expression of I-1c or I-1S67A enhanced steady-state phosphorylation of 2 key Ca2+-regulating sarcoplasmic reticulum enzymes. This was associated with increased contractile function in young animals but also with arrhythmias and cardiomyopathy after adrenergic stress and with aging. These data should be considered in the development of novel therapies for heart failure.
Figures




References
-
- Bristow MR. beta-adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101(5):558–569. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

