Differential activation of the periaqueductal gray by mild anxiogenic stress at different stages of the estrous cycle in female rats
- PMID: 20072120
- PMCID: PMC3055401
- DOI: 10.1038/npp.2009.222
Differential activation of the periaqueductal gray by mild anxiogenic stress at different stages of the estrous cycle in female rats
Abstract
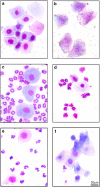
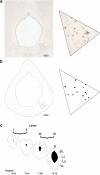
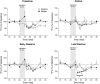
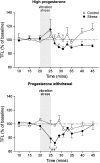
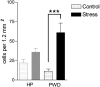
The effect of acute exposure to mild anxiogenic stress on cutaneous nociceptive threshold was investigated in female Wistar rats at different stages of the estrous cycle. Baseline tail flick latencies did not change significantly during the cycle. However after brief exposure to vibration stress (4 Hz for 5 min), rats in late diestrus, but not at other cycle stages, developed a hyperalgesia (decrease in tail flick latency). Animals in late diestrus revealed a more than fivefold increase in the density of Fos-like immunoreactive nuclei in the dorsolateral, lateral, and ventrolateral columns in the caudal half of the periaqueductal gray matter (PAG). There was no change in the density of Fos-like immunoreactive nuclei in the PAG in rats in estrus and early diestrus, although rats in proestrus showed a smaller (50%) but significant increase. Rats undergoing withdrawal from a progesterone dosing regimen (5 mg/kg i.p. twice daily for 6 days) designed to mimic the fall in progesterone that occurs naturally during late diestrus, exhibited a stress-induced hyperalgesia that was similar to animals in late diestrus and a significant increase in Fos-positive cells in the PAG. We suggest that falling levels of progesterone during late diestrus may be a predisposing factor for the development of stress-induced hyperalgesia, which is linked to differential activation of descending pain control circuits in the PAG. Similar changes in women, when progesterone levels fall during the late luteal phase of the menstrual cycle, may contribute to the development of premenstrual symptoms that include increased anxiety and hyperalgesia.
Figures
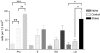



References
-
- Aloisi AM, Steenbergen HL, van de Poll NE, Farabollini F. Sex-dependent effects of restraint on nociception and pituitary-adrenal hormones in the rat. Physiol Behav. 1994;55:789–793. - PubMed
-
- Bajaj P, Arendt-Nielsen L, Bajaj P, Madsen H. Sensory changes during the ovulatory phase of the menstrual cycle in healthy women. Eur J Pain. 2001;5:135–144. - PubMed
-
- Bee LA, Dickenson AH. Descending facilitation from the brainstem determines behavioural and neuronal hypersensitivity following nerve injury and efficacy of pregabalin. Pain. 2008;140:209–223. - PubMed
-
- Beitz AJ. The organization of afferent projections to the midbrain periaqueductal gray of the rat. Neurosci. 1982;7:133–159. - PubMed
-
- Borta A, Schwarting RK. Inhibitory avoidance, pain reactivity, and plus-maze behavior in Wistar rats with high versus low rearing activity. Physiol Behav. 2005;84:387–396. - PubMed

